Research Article
Chris Packer
Chris Packer
Corresponding Author
Finca Botanica Aromatica,
Guayaquil, 090151, EC, Ecuador
E-mail: cpacker@youngliving.com, adabad@youngliving.com
Tel: +1 208 5300067
Adrian Abad
Tyler M. Wilson
Tyler M. Wilson
D. Gary Young Research Institute, Lehi, UT 84043,
USA
E mail: tywilson@youngliving.com
Brett J. Murphy
Brett J. Murphy
D. Gary Young Research Institute, Lehi, UT 84043,
USA
Tulio Orellana
Tulio Orellana
Finca Botanica Aromatica, Guayaquil,
090151, EC, Ecuador
Eugenio Caruajulca
Eugenio Caruajulca
Finca Botanica Aromatica,
Guayaquil, 090151, EC, Ecuador
Orlando Pacheco
Orlando Pacheco
Finca Botanica Aromatica,
Guayaquil, 090151, EC, Ecuador
Richard E. Carlson
Richard E. Carlson
D. Gary Young Research Institute, Lehi, UT 84043,
USA
Abstract
Lippia
alba (Mill.) N.E.Br. ex Britton & P. Wilson is a
medicinal plant known for its diverse therapeutic/ethnobotanical applications. This
study establishes the volatile profile (GC/MS) and stable isotope profiles of
prominent volatile compounds from distinct harvest times throughout the
cultivation season. Samples (n=48) were obtained through steam distillation from
plants harvested at two distinct times: morning (9:00 hours) and afternoon
(13:00 hours) over 8 weeks. Analysis by GC/MS identified the predominant compounds, including 6-methyl-5-hepten-2-one
(2.4-2.7%), nerol (2.0-2.3%), neral (25.9-26.3%), geraniol (15.2-16.2%),
geranial (29.8-29.9%), geranyl acetate (1.8-2.0%), β-elemene (1.3-1.5%),
(E)-caryophyllene (5.7-6.1%), germacrene D (5.2-5.7%), and (E)-α-bisabolene
(1.6-1.8%), which could classify this species in the Citral chemotype. While
overall oil yield showed no notable differences between harvest times,
variations were observed in specific compounds, including nerol, geranyl
acetate, β-elemene, germacrene D, and (E)-α-bisabolene. Additionally, stable isotope
values for the 3 most prominent compounds were assessed, revealing a negative
linear regression for geraniol (R2 =0.5). Overall, the findings
suggest that harvest time exerts a minimal impact on the oil's composition,
indicating potential flexibility in harvesting practices.
Abstract Keywords
Chemical profile, essential oil, ethnobotanical, Lippia alba, Verbenaceae, yield.
1.
Introduction
Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson is a small aromatic shrub that belongs to the Verbenaceae family and is known by the common names Incan melissa, Falsa melissa, Pronto alivio, Quita dolor, or Cidrela [1]. L. alba grows to 0.8 m tall, and its limbs have variable forms with a "pointed apex, cuneiform, or decumbent base, and serrated or crenated border” [2, 3]. This species is native to Central and South America [4] and is used traditionally throughout Latin America [5].
In various countries, the ethnobotanical applications of Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson span a range of ethnobotanical uses, underlining its versatility and importance in traditional medicine. In Brazil, both the leaves and roots of the plant have been employed for healing purposes. The leaves have been traditionally infused to treat conditions such as hypertension, stomach colic, nausea, and colds; they have also been applied externally for wound healing. In contrast, the roots have been predominantly used in infusions to address colds and coughs [6]. Mexico witnesses the utilization of its leaves in infusions aimed at alleviating gastrointestinal pain, vesicle discomfort, and gastritis [7, 8]. Similarly, in Guatemala, the leaves of the plant are essential; their decoctions and infusions have been used for conditions like coughs, skin diseases, flatulence, nausea, and headaches [9]. The versatility of Lippia alba is further highlighted in Colombia, where aqueous extracts of its leaves have been used as teas with properties ranging from antidiabetic and antispasmodic to diaphoretic, emmenagogue, and sedative [10]. In Peru, aqueous leaf extracts have been popularly consumed to alleviate migraine headaches [10]. Finally, in France, the plant's scope extends to its branches, which, along with the leaves, have been used in decoctions or infusions, primarily to combat conditions like insomnia and anxiety [11]. These applications accentuate the plant's widespread recognition and its pivotal role in various traditional medicinal practices. In Ecuador, the origin country of L. alba in this study, the leaves are cooked and used to treat cough, fever, headache, and stomachache. As part of a traditional Ecuadorian herbal mixture, L. alba is also used to treat diseases caused by climatic variations. The Shuar indigenous group has used this plant to relieve bone pain, stomach pain, cramps, and colic [12]. The ethnobotanical uses of this plant may begin to be elucidated by a study of the chemical constituents in the essential oil [13].
Lippia alba has been the focus of in vitro pharmacological studies investigating its extracts and fractions. These studies have evidenced antimicrobial activity against multidrug-resistant pathogens and antioxidant effect (DPPH assay) [3,14]. Additionally, extract from this species has demonstrated effective antimicrobial activity, particularly against Gram-positive bacteria [15]. On another note, the essential oil derived from the leaves of L. alba has shown significant antioxidant activity, even when compared with vitamin E, a well-known antioxidant [2].
Analyses of the essential oil composition derived from L. alba have revealed a diverse array of chemical profiles, indicative of seven unique chemotypes. These chemotypes are characterized by their principal compounds as follows: Chemotype I contains Citral, Linalool, and β-caryophyllene; Chemotype II is dominated by Tagetenone; Chemotype III features Limonene and Carvone; Chemotype IV is characterized by Myrcene; Chemotype V has γ-Terpinene as its major compound; Chemotype VI comprises Camphor and 1,8-Cineole; and Chemotype VII is highlighted by Estragole. Particularly, investigations focusing on chemotypes I and III have demonstrated a range of therapeutic effects, including antispasmodic, anxiolytic, and anti-inflammatory activities [4,16-19].
This study characterizes the volatile profile and distillation yield of
Lippia alba essential oil from Ecuador. To the best of the
authors' knowledge, this is the first attempt to establish its stable isotope
ratios.
This research will contribute to the
existing body of knowledge by providing insights into the extent to which
harvest time can impact the composition of essential oils, and by extension, provide
insight into best harvesting practices.
2. Materials
and methods
2.1
Plant material
Lippia alba was collected from February to April 2022 in Guayaquil, Ecuador (2°16'36.2"S 80°04'13.4"W). The first harvest was done in 7-month-old plants (Figure 1), with harvest and distillations in triplicate each week, for a total of 8 weeks. All the harvests were performed in the morning (09:00 hours) and in the afternoon (13:00 hours), at a height of 10 cm above the ground. Environmental conditions (relative humidity % and temperature) were recorded at each harvest time with Vantage Vue Weather Station (Davis Instruments, USA). The voucher sample of L. alba was deposited in the herbarium Universidad de Guayaquil (13.552 GUAY).
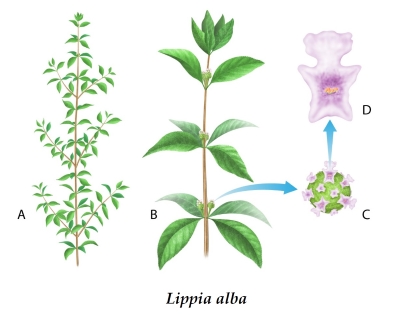
Figure 1. Botanical illustration of Lippia
alba species used in the study.
(A) Illustration of the overall plant,
showing the leaf pattern and branching structure. (B) Illustration of close
perspective and inflorescence. (C)/(D) Illustration of close/detailed
perspective of flowering structure(s). Illustrated by Rick Simonson, Science
Lab Studios, Inc. (Kearney, NE, USA).
2.2
Extraction method
The fresh plant material was chopped into small, non-uniform pieces,
and the essential oil was immediately extracted by steam distillation.
Considering the three harvests per week (and per harvest time), six
distillations were carried out per week, producing a total of 48 essential oil
samples (n = 48).
All distillations were carried out in a 250 L distillation chamber
(Albrigi Luigi S.R.L., Italy). Each batch of raw material was weighed before
being placed in the chambers to get 15 kg of plant material. The steam distillations
were carried out for 2 hours, and the essential oil was separated by a cooled
condenser. The essential oils were collected, filtered, and stored in sealed
amber vials at room temperature (25 °C) until analysis. The essential oil yield
was calculated by dividing the volume of the essential oil obtained by the mass
of the plant material before distillation.
2.3
Gas chromatographic analyses
The essential
oil compounds were separated, identified, and quantified using GC/MS using an
Agilent 7890B GC/5977B MSD (Agilent Technologies, Santa Clara, CA, USA) and
Agilent J&W DB-5, 60 m × 0.25 mm, 0.25 μm film thickness, fused silica
capillary column. Operating conditions: 0.1 μL of sample (20% soln. for
essential oils in methylene chloride), 150:1 split ratio, initial oven
temperature of 40 °C with an initial hold time of 5 min, oven ramp rate of 4.5
°C per minute to 310 °C with a hold time of 5 min, helium carrier gas. The
electron ionization energy was 70 eV, scan range 35–650 amu, scan rate 2.4
scans per second, source temperature 230 °C, and quadrupole temperature 150 °C.
Volatile compounds were identified using a combination of retention time data
from reference compounds, (MilliporeSigma, Sigma-Aldrich, St. Louis, MO, USA) the
Adams volatile oil library [20], and using
Chemstation library search in conjunction with retention indices.
The stable carbon
isotope ratios of L. alba essential oils were analyzed by GC/C/IRMS
using a Thermo TRACE 1310 GC coupled to a Thermo Delta V Advantage Isotope
Ratio Mass Spectrometer (ThermoFisher Scientific, Waltham, MA, USA), with an
Agilent J&W DB-5, 60 m × 0.25 mm, 0.25 μm film thickness, fused silica
capillary column.
Essential oil
samples were prepared for GC/IRMS analysis (13C/12C) as
follows: 35 mg of sample was weighed into a 2 mL clear glass vial and brought
up to 1 mL with hexane. A 90 μL aliquot was added to a second vial and the
final volume was brought to 1.0mL using hexane.
GC operating
conditions are as follows: splitless injection of 1 μL of sample with splitless
time set at 0.25 min., injection port 270 °C, initial oven temp. 40 °C with an
initial hold time of 5.0 min., oven ramp rate of 6.0 °C per min. to 250 °C with
a hold time of 2.0 min., then an oven ramp rate of 10.0 °C per minute to 310 °C
with a hold time of 7.0 min., helium carrier gas with constant flow 1.4 mL/min.
After passing through the capillary column, samples were sent through the
combustion reactor for 13C/12C analysis. The
combustion reactor temp. was set to 1000 °C and was conditioned with oxygen at
regular intervals.
To normalize IRMS results, reference materials were purchased from Dr. Arndt Schimmelmann at Indiana University and the United States Geological Survey (USGS) – Reston Stable Isotope Laboratory. δ13C isotope ratios are expressed relative to VPDB. The following three reference materials, along with their known values, were used to normalize results: hexadecane #C (USGS69), δ13C: −0.57‰; nonadecane #2, δ13C: −31.99‰; and tetradecanoic acid methyl ester #14M, δ13C: −29.98‰.
Samples were analyzed in quadruplicate to ensure repeatability. Isotope ratios were determined for the following prominent compounds: neral, geraniol, and geranial. δ13C values are reported with a standard deviation ≤ 0.3‰.
The statistical analysis to compare the effect of harvesting time on
essential oil yield and chemical profile was done with two independent sample t-tests
at a confidence level of 95%. For investigating the significance of trends in
stable isotope datasets, linear regressions were evaluated.
3. Results
and discussion
The information
presented in Table 1 contains data on environmental conditions at the time of
harvest and essential oil (EO) yield for each week of collection. The relative
standard deviation (RSD) is provided for the average essential oil yield per
week in the 8 weeks of the study.
Table
1.
Averages yield data from harvest time for each week, including essential oil yield
(mL), and calculated yield (mL/kg), Environmental conditions as average
relative humidity percentage and average temperature.
|
Harvest
time |
Week |
Avg.
relative humidity [%] |
Avg.
temperature [°C] |
Avg.
EO Yield [mL/kg] |
|
Morning |
1 |
84.7 |
25.5 |
2.9 |
|
2 |
89.3 |
25.5 |
2.6 |
|
|
3 |
80.0 |
30.0 |
2.6 |
|
|
4 |
69.7 |
32.9 |
3.0 |
|
|
5 |
87.0 |
28.4 |
2.6 |
|
|
6 |
77.7 |
29.6 |
2.5 |
|
|
7 |
81.0 |
27.5 |
3.1 |
|
|
8 |
78.7 |
30.7 |
3.3 |
|
|
Avg |
81.0 |
28.8 |
2.8 |
|
|
% RSD (n=8) |
|
|
10.9 |
|
|
Afternoon |
1 |
67.7 |
31.1 |
3.3 |
|
2 |
59.3 |
33.6 |
3.0 |
|
|
3 |
70.3 |
32.6 |
2.8 |
|
|
4 |
71.0 |
31.5 |
3.2 |
|
|
5 |
68.0 |
32.8 |
2.9 |
|
|
6 |
72.3 |
30.5 |
2.7 |
|
|
7 |
68.0 |
31.8 |
3.6 |
|
|
8 |
68.7 |
31.0 |
3.3 |
|
|
Avg |
68.2 |
31.9 |
3.1 |
|
|
% RSD (n=8) |
|
|
9.3 |
Table 1 shows an average essential oil yield for the morning harvest of 2.8 mL/kg and 3.1 mL/kg for the afternoon harvest. These results suggest no significant difference (p>0.05) in the essential oil yield of steam-distilled Lippia alba as a function of the harvest times selected for this study. Findings in the current study are in accordance with a prior investigation conducted on L. alba in Brazil, wherein no significant differences were observed in the essential oil yield at various harvest times [21]. However, a separate study focusing on the same species in Brazil, revealed contrasting outcomes, presenting differences in essential oil yield [22]. The authors of this latter study attributed their results to fluctuations in temperature and light intensity throughout the day. Furthermore, investigations encompassing distinct species have exhibited a similar tendency concerning essential oil yield, in which an increase or decrease is observed contingent upon the harvest time [23-26].
The information
presented in Table 2 shows the volatile compounds of L. alba essential
oil with the average relative area percentages from all samples in their
respective harvest times.
Table
2.
List of volatile compounds of L. alba essential oil in the morning (9:00
hours) and afternoon (13:00 hours) harvest times, GC/MS.
|
Compound
Name |
Relative
Area (%) |
||
|
KI |
Morning |
Afternoon |
|
|
(3Z)-Hexenol |
850 |
0.2 |
0.2 |
|
Camphene |
946 |
tr |
tr |
|
1-Octen-3-ol |
974 |
0.6 |
0.5 |
|
6-Methyl-5-hepten-2-one |
981 |
2.4 |
2.7 |
|
Dehydro-1,8-cineole |
988 |
0.2 |
0.1 |
|
3-Octanol |
988 |
0.1 |
0.1 |
|
(E)-β-Ocimene |
1044 |
0.2 |
0.1 |
|
Rosefuran |
1093* |
tr |
tr |
|
Linalool |
1095 |
0.8 |
0.8 |
|
Perillen |
1102 |
tr |
tr |
|
Exo-isocitral |
1140 |
tr |
tr |
|
Citronellal |
1148 |
0.1 |
0.2 |
|
Isoneral |
1160 |
0.7 |
0.7 |
|
Borneol |
1165 |
0.2 |
0.2 |
|
Rosefuran epoxide |
1173 |
0.2 |
0.2 |
|
(E)-Isocitral |
1177 |
1.3 |
1.2 |
|
α-Terpineol |
1186 |
tr |
tr |
|
Nerol |
1227 |
2.3 |
2.0 |
|
(Z)-Isogeraniol |
1230* |
0.3 |
0.2 |
|
Neral |
1235 |
26.3 |
25.9 |
|
Geraniol |
1249 |
16.2 |
15.2 |
|
Geranial |
1264 |
29.9 |
29.8 |
|
Neryl acetate |
1359 |
0.1 |
0.1 |
|
Geranyl acetate |
1379 |
1.8 |
2.0 |
|
β-Bourbonene |
1387 |
0.1 |
0.1 |
|
β-Elemene |
1389 |
1.3 |
1.5 |
|
(E)-Caryophyllene |
1417 |
5.7 |
6.1 |
|
β-Copaene |
1430 |
0.2 |
0.2 |
|
α-Humulene |
1452 |
tr |
tr |
|
Allo-aromadendrene |
1458 |
0.2 |
0.4 |
|
γ-Muurolene |
1478 |
tr |
tr |
|
Germacrene D |
1480 |
5.2 |
5.7 |
|
α-Muurolene |
1500 |
0.3 |
0.4 |
|
(E)-α-Bisabolene |
1536* |
1.6 |
1.8 |
|
Caryophyllene oxide |
1582 |
0.1 |
0.2 |
|
Compound Classes |
|
|
|
|
0.5 |
0.4 |
||
|
Oxygenated
monoterpenes |
78.5 |
76.5 |
|
|
Sesquiterpene
hydrocarbons |
14.6 |
16.2 |
|
|
Oxygenated
sesquiterpenes |
0.1 |
0.2 |
|
|
Other
compounds |
5.2 |
5.6 |
|
|
Total |
98.9 |
98.9 |
|
Note:
Compounds detected with values less than 0.1% are denoted as trace (tr).
Unidentified compounds of less than 0.5% are not included. KI is the Kovat’s
Index previously calculated by Robert Adams using a linear calculation on a
DB-5 column [20]. *KI not previously
calculated [20] and manual calculation
performed using alkane standards. Data obtained from GC/MS analysis.
In an effort to
identify trends, the ten major compounds present in the essential oil of L.
alba were considered. These ten compounds are 6-methyl-5-hepten-2-one,
nerol, neral, geraniol, geranial, geranyl acetate, β-elemene, (E)-caryophyllene,
germacrene D, and (E)-α-bisabolene. The high content of neral (25.9-26.3%) and geranial
(29.8-29.9%) in these results suggests that the L. alba species in
this report belong to the Citral chemotype or chemotype I. Considering this
chemotype, it is interesting to note the high geraniol percentage (15.2-16.2%) in
the essential oil samples. Other studies on Lippia alba Citral chemotype
have shown geraniol percentages ranging from not detected to 7.6% [27-31]. Essential oils
with a high concentration of Citral, such as Citrus limon, Litsea
cubeba, Cinnamomum camphora, and Cymbopogon flexuosus have
garnered considerable attention in recent literature due to their array of
therapeutic, culinary, and industrial applications [32-35]. For instance, the escalating demand for
natural and organic products has elevated the prominence of these Citral-rich essential
oils as they are frequently integrated into cosmetics, perfumes, aromatherapy,
and even in the food and beverage sector [32, 34,
36]. Their economic relevance extends beyond consumer products, as they
are applicable in sustainable agricultural practices, acting as potential
biopesticides, thus reducing the dependence on synthetic chemicals [37, 38]. Similarly,
L. alba from our study, which boasts high concentrations of Citral,
holds significant potential in various therapeutic, culinary, and industrial
applications, following the trend observed in other Citral-rich species as
previously highlighted.
The essential
oil obtained from L. alba showed quantitative significant differences (p<0.05)
in five compounds which are presented in Table 3, comparing the morning and
afternoon harvest times.
Table
3. Main volatile compounds of L. alba essential
oil with significant differences between harvest times.
|
Compound |
Significant
differences (p<0.05) |
|
Nerol |
Higher
in the morning (2.3%) compared to the afternoon (2.0%) |
|
Geranyl
acetate |
Lower
in the morning (1.8%) compared to the afternoon (2.0%) |
|
β-elemene |
Lower
in the morning (1.3%) compared to the afternoon (1.5%) |
|
Germacrene
D |
Lower
in the morning (5.2%) compared to the afternoon (5.7%) |
|
Lower
in the morning (1.6%) compared to the afternoon (1.8%) |
Although no literature was found comparing harvest time with the Citral chemotype of L. alba, the current findings diverge from those reported in the carvone chemotype L. alba from Brazil, where, based on harvest times, significant differences were observed in the percentages of carvone (26.7-54.9%), limonene (20.45-28.66%), sabinene (1.38-2.25%), and linalool (1.18-1.72%) [21]. The slight variability in the concentration of the five compounds (Table 3) in this study may have limited implications in terms of the biological properties of L. alba essential oil. The magnitude of the differences observed indicates that this influence is likely minimal in the harvest times (9:00 hours and 13:00 hours) used in this study.
Stable isotope
values were determined for the 3 most prominent compounds (neral, geraniol,
geranial) for each sample (n = 48). Average stable isotope values, and
associated data, for both morning and afternoon samples are detailed in Table 4.
Table
4. Stable
isotope (δ13C) values (average, minimum, maximum, standard
deviation) for neral, geraniol, and geranial for both a.m. and p.m. collection
times spanning 8 weeks (n = 48). Samples were analyzed in quadruplicate
and standard deviations for repeat injections are ≤ 0.3‰. δ13C
isotope ratios are expressed relative to VPDB.
|
Compound
Name |
Morning/ Afternoon |
Avg. Value (δ13C ‰) |
Min. Value (δ13C
‰) |
Max. Value (δ13C
‰) |
Std. Dev. |
|
Neral |
Morning |
-26.332 |
-27.477 |
-25.062 |
0.691 |
|
Neral |
Afternoon |
-26.512 |
-28.262 |
-24.665 |
0.779 |
|
Geraniol |
Morning |
-30.685 |
-33.413 |
-25.362 |
1.992 |
|
Geraniol |
Afternoon |
-31.138 |
-33.900 |
-25.862 |
1.709 |
|
Geranial |
Morning |
-29.245 |
-30.663 |
-27.625 |
0.788 |
|
Geranial |
Afternoon |
-29.451 |
-30.563 |
-27.835 |
0.738 |
When considering the chemical structure of terpenes, and particularly those investigated in the current study for stable isotope analysis, plausible elements to analyze include carbon, hydrogen, and oxygen. However, carbon (δ13C) was selected due to inherent repeatability upon repeat injections of the same sample, where standard deviations for quadruplicate analysis range from neral (0.04 to 0.26), geraniol (0.05 to 0.22), and geranial (0.01 to 0.20). Given the confidence in carbon (δ13C) values, any apparent shifts or trends in the data may carry significance. When considering both morning and afternoon data points for Citral (neral + geranial), there appears to be a positive trend/slope for neral and a negative trend/slope for geranial from February to April (Figure 2). However, the trends for both neral (R2 = 0.1) and geranial (R2 = 0.3) are not statistically significant, due to the broad scatter along the regression line. However, the negative linear regression for geraniol (R2 = 0.5) is significant. Traditional taxonomy and plant identification often view chemotypes as identical, but stable isotope analysis offers a deeper understanding of the plant's molecular processes. In recent years, the significance of stable isotope analysis in authenticating the origin and quality of essential oils has gained increasing attention. Not only does this technique allow for the differentiation of products based on geographical and temporal variables, but it also aids in distinguishing genuine essential oils from their adulterated counterparts [39-41]. A key component in the interpretation of such data is the existence of comprehensive databases, which enable the contextualization and verification of results. The present study, by contributing to these databases, amplifies the robustness of stable isotope analysis, specifically for Lippia alba Citral chemotype to authenticate the origin and quality of the species. Future studies with additional samples collected over a longer range of time between the hours of collection could illuminate the significance of trends in δ13C values.
Figure
2. Scatter
plot of morning and afternoon values (δ13C) for neral, geraniol, and
geranial, for the 8 weeks.
4. Conclusions
This study was
conducted to determine how harvest time could influence the yield (volatile
profile and stable isotope profile of prominent compounds) of Lippia alba
essential oil. Results revealed L. alba from this study belongs to Citral
chemotype because of the high content of neral (25.9-26.3%) and geranial
(29.8-29.9%). Given the recent attention towards Citral-rich essential oils for
various applications, our findings on L. alba's high Citral
concentration add to the existing body of knowledge. Findings herein demonstrated
no significant differences in essential oil yield (p>0.05), and significant
differences (p<0.05) in five compounds, nerol
(2.0-2.3%), geranyl acetate (1.8-2.0%), β-elemene (1.3-1.5%), germacrene D
(5.2-5.7%), and (E)-α-bisabolene (1.6-1.8%). Therefore, these results
suggest that harvest time might have a minor impact on the overall chemical
profile of L. alba essential oil, providing some flexibility in harvest
practices. However, it's crucial to consider that the current study was limited
to examining the effect of harvest time alone, and many other biotic and
abiotic factors could influence the composition and yield of the essential oil.
Stable isotope values (δ13C) for neral, geraniol, and geranial are
characteristic of other C4 carbon sequestering plants and only the linear
regression for geraniol is significant in this study (R2 = 0.5). Emphasizing
the importance of stable isotope analysis, our research not only contributes to
the verification of essential oil origin and quality but also adds the existing
databases used for these authentications. Future lines of research could
include exploring how other environmental and agronomic factors, such as soil
conditions, water availability, and temperature, can influence both the
composition and stable isotope profile of L. alba essential oil.
Moreover, a more detailed analysis of diurnal variability in essential oil
composition could be undertaken, with additional sampling points throughout the
day and for longer than 8 weeks.
Authors’ contributions
Conceptualization,
C.P.; Methodology, C.P., A.A., T.M.W., and B.J.M.; Software, C.P., A.A., and
T.M.W.; Validation, C.P. and A.A; Formal Analysis (GC/MS, GC/IRMS), C.P., A.A.,
T.M.W., T.O., and B.J.M.; Investigation, C.P. and A.A.; Resources, C.P., E.C., O.P.;
Data Curation, C.P., A.A., T.M.W, and B.J.M..; Writing – Original Draft, C.P., A.A.,
T.M.W., and B.J.M; Writing – Review & Editing, C.P., A.A., T.M.W., R.E.C., B.J.M,
E.C., T.O., and O.P.
Acknowledgements
The authors want
to thank the D. Gary Young Research Institute and Finca Botanica Aromatica, for
providing support for this project. Appreciation would also like to be extended
to Rick Simonson (Science Lab Studios).
Funding
This research was funded by Young Living Essential Oils
Conflicts of interest
The authors declare no conflict of interest. The funding entity had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
1.
Ministerio
de Salud y Protección Social. Vademécum colombiano de plantas medicinales,
Bogotá, Colombia: Imprenta nacional de Colombia, 2008.
2.
Stashenko, E.; Jaramillo, B.; Martı́nez,
J. Comparison of different extraction methods for the analysis of volatile
secondary metabolites of Lippia alba (Mill.) NE Brown, grown in
Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A.
2004, 1025(1), 93-103.
3.
Joshi, A.; Prakash, O.; Pant, A.; Kumar,
R.; Negi, M. Chemical Analysis and Antioxidant Activity of Essential Oils of
Two Morphotypes of Lippia Alba (Mill.) NE Br. Ex Britton & P. Wilson
(Verbenaceae). J. Essent. Oil Bear. Plants 2018, 21(3), 687–700.
4.
Hennebelle, T.; Sahpaz, S.; Joseph, H.;
Bailleul, F. Ethnopharmacology of Lippia Alba. J. Ethnopharmacol. 2008,
116 (2), 211–222.
5.
Albuquerque, U.; Patil, U.; Máthé, Á.
Medicinal and Aromatic Plants of South America, Springer, Brazil, 2018.
6.
Di Stasi, L.; Oliveira, G.; Carvalhaes,
M.; Queiroz-Junior, M.; Tien, O.; Kakinami, S.; Reis, M. medicinal plants
popularly used in the brazilian tropical atlantic forest. Fitoterapia. 2002, 73
(1), 69–91.
7.
Heinrich, M.; Rimpler, H.; Barrera, N.A.
Indigenous phytotherapy of gastrointestinal disorders in a lowland Mixe
community (Oaxaca, Mexico): Ethnopharmacologic evaluation. J. Ethnopharmacol. 1992,
36 (1), 63–80.
8.
Zamora-Martínez, M.; de Pascual Pola, C.
Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz,
Mexico. J. Ethnopharmacol. 1992, 35 (3), 229–257.
9.
Girón, L.; Freire, V.; Alonzo, A.;
Cáceres, A. Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala.
J. Ethnopharmacol. 1991, 34 (2–3), 173–187.
10.
Duke, J.; Bogenschutz-Godwin, M.;
Ottesen, A. Duke's handbook of
medicinal plants of Latin America. CRC press: Boca Raton, FL, USA, 2009.
11.
Tareau, M.; Palisse, M.; Odonne, G. As
Vivid as a weed. medicinal and cosmetic plant uses amongst the urban youth in
French Guiana. J. Ethnopharmacol. 2017, 203, 200–213.
12.
De
la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M. J.; Balslev, H. Enciclopedia
de Las Plantas Útiles Del Ecuador (Con Extracto de Datos); Herbario QCA de la
Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del
Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la
Universidad de Aarhus, Ecuador, 2008.
13.
Haro, J.; Castillo, G.; Martínez, M.;
Espinosa, H. Clove essential oil (Syzygium
aromaticum L. Myrtaceae): Extraction, chemical composition, food
applications, and essential bioactivity for human health. Molecules. 2021,
26(21), 6387.
14.
Teixeira de Oliveira, G.; Siqueira, J.; Lima,
W.; Ferreira, L.; Duarte-Almeida, J.; Alves, Santos Lima, L. Phytochemical
characterisation and bioprospection for antibacterial and antioxidant
activities of Lippia alba Brown ex
Britton & Wilson (Verbenaceae). Nat. Prod. Res. 2018, 32(6), 723-731.
15.
Machado, T.; Nogueira, N.; de Cássia, R;
de Sousa, C.; Batista, V. The antimicrobial efficacy of Lippia alba essential oil and its interaction with food
ingredients. Braz. J. Microbiol. 2014, 45(2), 699-705.
16.
Blanco, M.; Colareda, G.; van Baren, C.;
Bandoni, A.; Ringuelet, J.; Consolini, A. Antispasmodic effects and composition
of the essential oils from two South American chemotypes of Lippia Alba.
J. Ethnopharmacol. 2013, 149 (3), 803–809.
17.
Hatano, V.; Torricelli, A.; Giassi, A.;
Coslope, L.; Viana, M. Anxiolytic effects of repeated treatment with an
essential oil from Lippia Alba and (R)-(-)-carvone in the elevated T-Maze.
Braz. J. Med. Biol. Res. 2012, 45, 238–243.
18.
Junior, G.; de Abreu, M.; da Rosa, J.; Pinheiro,
C.; Heinzmann, B.; Caron, B.; Baldisserotto, B.; Barcellos, L. Lippia Alba
and Aloysia triphylla essential oils
are anxiolytic without inducing Aversiveness in fish. Aquaculture. 2018, 482,
49–56.
19.
Sepúlveda-Arias, J.; Veloza, L.;
Escobar, L.; Orozco, L.; Lopera, I. Anti-Inflammatory effects of the main
constituents and epoxides derived from the essential oils obtained from Tagetes
Lucida, Cymbopogon Citratus, Lippia Alba and Eucalyptus Citriodora. J.
Essent. Oil Res. 2013, 25 (3), 186–193.
20.
Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass
Spectrometry, 4th Edn.; Allured Publ.: Carol Stream, IL, USA,
2007.
21.
Ehlert, P.; Ming, L.; Marques, M.;
Fenandes, D.; Rocha, W.; Luz, J.; Silva, R. Influence of harvest time on the
yield and composition of essential oil from the Brazilian"
erva-cidreira"[Lippia alba (Mill.) NE Br.]. Rev. Bras. Pl. Med. 2013,
15, 72-77.
22.
dos Santos, M.; Innecco, R.; Soares, A.;
Anatomic characterization of secretory structures and essential oil production
of Lippia alba (Mill.) N.E. Brown in relation to harvest times in the
dry and rainy seasons. Revista Ciência Agronômica 2004, 35, 377-383.
23.
de
Oliveira, A.; Jezler, C.; Oliveira, R.; Mielke, M.; Costa, L. Determinação do
tempo de hidrodestilação e do horário de colheita no óleo essencial de menta.
Hortic. Bras. 2012, 30 (1), 155-159.
24.
Pinheiro, M.; Ribeiro J.; Meira, M.;
Figueiredo, L.; Martins, E. Essential oil content of pepper-rosmarin as a
function of harvest time. Cienc.
Rural. 2011, 41 (7), 1166-1169.
25.
Silva, E.; Silva, V.; Alves, C.; Alves,
J.; Souchie, E.; Barbosa, L. Harvest time on the content and chemical composition
of essential oil from leaves of guava. Cienc. Rural 2016, 46 (10), 1771-1776.
26.
Salehi, A.; Hazrati, S. How essential
oil content and composition fluctuate in German chamomile flowers during the
day?. J. Essent. Oil Bear. Plant. 2017, 20(3), 622-631.
27.
Veras,
H.; Campos, A.; Rodrigues, F.; Botelho, M.; Coutinho, H.; Costa, J.; Lippia
alba (mill.) NE essential oil interfere with
aminoglycosides effect against Staphylococcus
aureus. J. Essent. Oil Bear. 2011, 14(5), 574-581.
28.
Porfírio, E.; Melo, H.; Gomes, A.; Arruda,
T.; Gomes, G.; de Carvalho, M.; Costa, R.; Catunda, F. In vitro antibacterial
and antibiofilm activity of Lippia alba essential oil, citral, and
carvone against Staphylococcus aureus.
Sci. World. J. 2017, 2017, 1-7.
29.
López, M.; Stashenko, E.; Fuentes, J.
Chemical composition and antigenotoxic properties of Lippia alba
essential oils. Genet. Mol. Biol. 2011, 34 (3), 479-488.
30.
Moreno, É.; Leal, S.; Stashenko, E.; García,
L. Induction of programmed cell death in Trypanosoma
cruzi by Lippia alba essential oils and their major and synergistic
terpenes (citral, limonene and caryophyllene oxide). BMC Complement Altern Med.
2018, 18(1), 1-16.
31.
da Silva, A.; da Silva, D.; Figueiredo,
P.; Sarrazin, S.; Bouillet, L.; de Oliveira, R.; Maia, J.; Mourao, R. Seasonal
and circadian evaluation of a citral-chemotype from Lippia alba
essential oil displaying antibacterial activity. Biochem. Syst. Ecol. 2019, 85,
35-42.
33.
Yuxiang, Z.; Jianping W.; Hong C.; Zihan
S.; Hong G.; Yahong Y.; Tianli Y. Antibacterial activity of essential oils
against Stenotrophomonas maltophilia and the effect of Citral on cell
membrane. LWT. 2020, 117, 108667.
34.
Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.;
Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical composition and antioxidant
activity of the essential oils of Citral-Rich chemotype Cinnamomum camphora
and Cinnamomum bodinieri. Molecules. 2022, 27, 7356.
35.
Gao, S.; Liu, G.; Li, J.; Chen, J.; Li,
L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.; Zhang, S. Antimicrobial activity
of lemongrass essential oil (Cymbopogon flexuosus) and its active
component Citral against dual-species biofilms of Staphylococcus aureus
and Candida species. Front. Cell. Infect. Microbiol. 2020, 10, 603858.
36.
Hirai, M.; Ota, Y.; Ito, M. Diversity in
principal constituents of plants with a lemony scent and the predominance of
citral. J. Nat. Med. 2022, 76, 254-258.
37.
Moustafa, M.; Awad, M.; Amer, A.;
Hassan, N.; Ibrahim, E.; Ali, H.; Akrami, M.; Salem, M. Insecticidal activity
of lemongrass essential oil as an eco-friendly agent against the black Cutworm Agrotis
ipsilon (Lepidoptera: Noctuidae). Insects. 2021, 12(8), 737.
38.
Moustafa, M.; Hassan, N.; Alfuhaid, N.;
Amer, A.; Awad, M. Insights into the toxicity, biochemical activity, and
molecular docking of Cymbopogon citratus essential oils and Citral on Spodoptera
littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116(4),
1185–1195.
39.
Wilson, T.M.; Murphy, B.J.; Abad, A.;
Packer, C.; Poulson, A.; Carlson, R.E. Essential oil composition and stable
isotope profile of cultivated Ocimum campechianum Mill. (Lamiaceae) from
Peru. Molecules. 2022, 27, 2777.
40. Murphy, B.J.; Carlson, R.E.; Howa, J.D.; Wilson, T.M.; Buch, R.M. Determining the authenticity of methyl salicylate in Gaultheria procumbens L. and Betula lenta L. essential oils using isotope ratio mass spectrometry. J. Essent. Oil Res. 2021, 33(5), 442-451.
41. Tiên, T.K.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC. 2015, 66 (2015), 146-157.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
Lippia
alba (Mill.) N.E.Br. ex Britton & P. Wilson is a
medicinal plant known for its diverse therapeutic/ethnobotanical applications. This
study establishes the volatile profile (GC/MS) and stable isotope profiles of
prominent volatile compounds from distinct harvest times throughout the
cultivation season. Samples (n=48) were obtained through steam distillation from
plants harvested at two distinct times: morning (9:00 hours) and afternoon
(13:00 hours) over 8 weeks. Analysis by GC/MS identified the predominant compounds, including 6-methyl-5-hepten-2-one
(2.4-2.7%), nerol (2.0-2.3%), neral (25.9-26.3%), geraniol (15.2-16.2%),
geranial (29.8-29.9%), geranyl acetate (1.8-2.0%), β-elemene (1.3-1.5%),
(E)-caryophyllene (5.7-6.1%), germacrene D (5.2-5.7%), and (E)-α-bisabolene
(1.6-1.8%), which could classify this species in the Citral chemotype. While
overall oil yield showed no notable differences between harvest times,
variations were observed in specific compounds, including nerol, geranyl
acetate, β-elemene, germacrene D, and (E)-α-bisabolene. Additionally, stable isotope
values for the 3 most prominent compounds were assessed, revealing a negative
linear regression for geraniol (R2 =0.5). Overall, the findings
suggest that harvest time exerts a minimal impact on the oil's composition,
indicating potential flexibility in harvesting practices.
Abstract Keywords
Chemical profile, essential oil, ethnobotanical, Lippia alba, Verbenaceae, yield.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


