Research Article
Abdu Zakari
Abdu Zakari
Corresponding Author
Department of Chemical Sciences, Federal
University of Kashere, P.M.B 0182, Gombe – Nigeria.
E-mail: abdu.zakari@fukashere.edu.ng, zabdu70@gmail.com
Tel: +2348136202778
Said Jibrin
Said Jibrin
Department of Chemical Sciences, Federal
University of Kashere, P.M.B 0182, Gombe – Nigeria.
Fatope Majekodunmi Oladeji
Fatope Majekodunmi Oladeji
1.
Department of Chemistry, Sultan
Qaboos University, PO Box 36, Al-Khod, Muscat, Oman
Mohammed Hassan Shaggal
Mohammed Hassan Shaggal
1. Department of Chemistry, Modibbo Adama University of Technology,
P.M.B 2070, Yola –Nigeria
Andrew Sule
Andrew Sule
1.
Department of Medicinal Plants Research and Traditional Medicine,
National Institute for Pharmaceutical Research and Development (NIPRD), Idu–Abuja, Nigeria
Abstract
From the Stems
of Bark of Echinaceae angustifolia DC
three known triterpenes
3a,5,5b,8,8,11a-hexamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysene-9-yl
acetate (lupeol acetate),
4,4,6a,6b,8a,10,11,14b,octamethyl1,1,2,3,4,4a,5,6,6a,6b,7,8,8a, 9,10,
11,12,12a,14,14a,14b-icosahydropicen-3-yl acetate (derivative of β-amyrin and
9-hydroxy-1-isopropenyl-5a,5b,8,8,11a-pentamethyl-icosahydro-cyclopenta[a]chrysene-3a-carboxylic
acid (betulinic acid), labelled as Ea-7-38, Ea-9-10 and Ea-12-85) were isolated
and characterized. All isolates were tested for their cytotoxicities against Artemia salina (brine shrimp larvae).
Compound Ea-12-85 exhibited potent cytotoxic activity against the Artemia salina, Ea-7-38, Ea-9-10 were
found to be non-toxic in the cytotoxicity test. The result of the study has
justified the claim of the traditional medicine practitioners in Girei for the
treatment of complicated malaria disease using the stem bark of E. angustifolia DC.
Abstract Keywords
Isolation, 2D NMR,
structural elucidation, triterpene, ethnomedicinal, Echinaceae
angustifolia DC
1. Introduction
Echinacea angustifolia is a herbaceous, drought-tolerant perennial
plant growing up to 140 cm or 4 feet, in height. It grows from a
taproot and has an erect
unbranched stem that is unbranched. Both the basal and cauline (stem)
leaves are arranged alternately. The leaves are normally
hairy with a rough texture, having uniseriate trichomes (1–4 rings of cells) but
sometimes they lack hairs. The basal leaves and the lower stem leaves
have petioles, and as the leaves progress up the stem
the petioles often decrease in length. The leaf blades in different species
may have one, three or five nerves.
Bioactivity Studies of
Echinacea Species revealed promising results as reported that
aerial extract of E. purpurea alters the clinical course of influenza
infection in mice and has antiviral activity [1].
Wu et al., reported that the concentrations of standardized purified dry
extract from Echinacea angustifolia showed positive effect on
proliferation and interferon gamma secretion of peripheral blood mononuclear
cells in dairy heifers [2]. It was reported
that Echinacea angustifolia extract can stimulate mammary epithelial
cell physiology and may be considered a candidate to support mammary gland
activity during a mammogenetic and lactogenetic state [3].
Also, Echinacea angustifolia has an immunomodulatory action on sheep
neutrophils [4]. The study revealed that E.
angustifolia extract significantly inhibited adhesion and superoxide
production induced by Phorbol
Myristate Acetate (PMA) that is known to increase the exposure of active
b2-integrins on cell surface and to activate ROS generation by NADPH oxidase. Spelman et al.,
demonstrated that undeca-2E-ene-8,10-diynoic acid isobutylamide an Echinacea
angustifolia-derived alkylamide, inhibits IL-2 secretion in Jurkat T cells
through Peroxisome Proliferator-Activated Receptor gamma (PPARϒ)
activity at low micromolar concentrations (330 ng/mL) [5].
Moreover, Nyalambisa reported that the root and leaf of Echinacea species
contain volatile oils which varied in their yield and chemical compositions [6]. The essential root oil is non-toxic orally and it
demonstrated significant anti-inflammatory and analgesic activities in
laboratory animals. The aim of this study is to investigate the bioactive
compounds responsible for the observed bioactivity of the stem bark of E
angustifolia DC. The specific objectives of the study are to; i) isolate
and characterize the pure compounds from the stem bark extracts and ii)
investigate the cytotoxicity of the pure compounds.
2. Materials and methods
2.1
Chemicals/reagents
Good
quality reagents and solvents of analytical grade were purchased, redistilled
and used throughout the laboratory work. Precoated plates (PK6F silica Gel 60Ao)
with fluorescent indicator size 20cm by 20cm (1000µm thickness) were used for
preparatory thin-layer chromatography. Sephadex LH-20 from Pharmacia
17-00-0-01, 25-100 micron were used for some highly Polar fractions.
2.2 Collection and preparation of plant materials
The
stem-bark of the plants under investigation was collected from Girei Local
Government Area of Adamawa State. Botanist in the Department of Biological
Sciences Federal University of Kashere identified the plants, the plants
specimens and voucher number (F.H.I 56535) were kept in the Herbarium. The
plant samples were washed with distilled water, air-dried, ground to fine
powder and weighed.
2.3 Extraction
Soxhlet extraction method was employed for the
initial extraction to obtain the plant extracts for phytochemical screening.
For the large scale extraction powdered sample of Echinaceae angusifolia DC (1.9Kg) was successively extracted
with dichloromethane DCM, then methanol using Soxhlet apparatus and concentrated using Rotary film evaporator.
The yield of the extracts was recorded.
2.4 Phytochemical screening
Standard
method described by Usman et al., [20] was used to test for the presence of
phytochemical compounds (saponins, sterols, terpenoids, glycosides,
phlobatannins, resins, flavonoids, phenols, alkaloids, and carbohydrates) in
the extracts.
2.5 Brine
shrimp lethality assay of isolated compounds
Artemia salina eggs were
added into a hatching chamber three quarter filled with ocean seawater. The chamber was kept in an open space for 24 h, after
which the eggs hatched into Artemia salina larvae. Five concentrations
(1000, 500, 250, 125, and 62.5) in µg/ml were prepared in test vials for each
compound in triplicate. To each sample vial, a drop of DMSO solvent was added
followed by 4ml ocean water. Two control groups were prepared, a positive
control vial contained 4ml of methanol and a negative control vial contained
4mL of distilled water. Ten (10) larvae were introduced into each vial using a
Pasteur pipette and allowed to stand for 24 h, the number of survivals were
counted against a lighted background and recorded. Nauplii (larvae) were
considered dead if they were lying immobile at the bottom of the vials, and the
percentage of deaths at each dose and in the control were determined. Microsoft
Excel spreadsheet application was used to formulate the regression equations
from the data of mean results of percentage mortality of the brine shrimp
versus the log of concentrations to base ten. These equations were later used
to calculate LC50 values for the compounds tested noting that value
greater than 1000 µg/mL suggests a nontoxic compound [7].
2.6
Column chromatography of
the extract
2.7 Preparation of sample for spectroscopic
analysis
2.8 Spectroscopic measurement
Spectra
in the 2D NMR analysis obtained include Correlation Spectroscopy (1H-1H
COSY), Heteronuclear Single Quantum Correlation (HSQC), Heteronuclear Multiple
Bond Correlation (HMBC), Nuclear Overhauser Effect Spectroscopy (NOESY),
Rotational Frame Overhauser Effect Spectroscopy (ROESY) and 13C DEPT
(90, 45, and 135) for the pure samples.
3. Results and discussion
3.1 Characterization of compounds
The
phytochemical screening of the dichloromethane and methanol extracts of Echinaceae angusifolia
DC (Compositae) showed
the presence of some secondary metabolites with the methanol extracts
indicating the presence of most of the secondary metabolites (Table 1). Secondary metabolites
have been shown to exhibit therapeutic activities, biological functions and
pharmacological properties as supported by [9].
Alkaloids were reported to show antiplasmodial
properties, analgesic, cytotoxic and anti-inflammatory activities [10]. Tannins have been
reported to show anti-oxidant activities and inhibit the growth of
microorganisms [11].
Table 1. Phytochemical
Screening Result of Solvent Extracts of Echinaceae
angustifolia DC
|
Phytochemical Constituents |
DCM |
MeOH |
|
Indole alkaloid |
+ |
+ |
|
Tropane alkaloids |
- |
+ |
|
Quinoline alkaloids |
+ |
+ |
|
Morphine alkaloids |
- |
- |
|
Steroids |
++ |
+++ |
|
Flavonoids |
+++ |
++ |
|
Saponins |
+++ |
+++ |
|
Tannins |
+ |
- |
|
Terpenes |
++ |
+++ |
|
Phenols |
++ |
+ |
|
Carbohydrates |
+++ |
+++ |
|
Anthraquinones |
- |
++ |
|
Resin |
- |
- |
|
Cardiac glycosides |
+ |
+ |
|
Key: - = absent, + = faintly present, ++ =
Present, +++ = Highly present |
||
Table 2. Physical Properties of
Isolated Compounds
|
Solvent ratio |
Ordinary light |
UV light |
Dodeca-molybdophosphoric acid (MPA) Spray |
M P(oC) |
Rf value |
MW (g/mol) |
|
|
Hex/ DCM 7:3 |
White |
Green |
Reddish brown |
367-368 |
0.42 |
468 |
|
|
Ea-9-10 |
Hex/ DCM 7:3 |
White |
Green |
Reddish brown |
693-694 |
0.51 |
468 |
|
Ea-12-85 |
DCM/ CHCl3 4:1 |
White |
Green |
Brown |
520-521 |
0.50 |
456 |
Table 3. 13C and 1H NMR
data (Chloroform-d1 700 MHz for 1H and 176 MHz 13C) for
Compound Ea-7-38
|
ẟCa |
ẟH/
Multiplicity |
J
value (Hz) |
|
|
1 |
39.99
CH2 |
1.64
t |
2.8 |
|
2 |
38.03
CH2 |
1.03,
q |
3.8 |
|
80.98 CH |
46, dd |
3.3, 4.3 |
|
|
4 |
42.99
C |
- |
- |
|
5 |
48.00
CH |
2.39,
ddd |
6.9,7.5,
7.9, |
|
6 |
35.56
CH2 |
1.58,
q |
5.6 |
|
7 |
34.20
CH2 |
1.6,
t |
5.3 |
|
8 |
37.08
C |
- |
- |
|
9 |
48.68
CH |
1.06,
t |
7.2 |
|
10 |
42.81
C |
- |
- |
|
11 |
29.82
CH2 |
1.07,
q |
1.4 |
|
12 |
27.92
CH2 |
1.37,
q |
8.5 |
|
13 |
27.94
CH |
1.04,
q |
3.7 |
|
14 |
21.33
CH |
2.07,
t |
1.3 |
|
15 |
21.31CH |
2.09,
q |
9.4 |
|
16 |
25.08
CH2 |
1.37,
d |
8.5 |
|
17 |
21.30CH |
- |
- |
|
18 |
21.29CH |
1.06,
t |
8.9 |
|
19 |
48.28
CH |
1.61,
q |
4.8 |
|
20 |
150.96
C |
- |
- |
|
21 |
23.71
CH2 |
1.69,
q |
5.6 |
|
22 |
21.32
CH2 |
1.66,
t |
6.4 |
|
23 |
19.28
CH3 |
1.69,
s |
- |
|
24 |
17.99
CH3 |
0.79,
s |
- |
|
25 |
16.49
CH3 |
0.79,
s |
- |
|
26 |
16.35
CH3 |
1.03,
s |
- |
|
27 |
16.17
CH3 |
0.79,
s |
- |
|
28 |
15.97
CH3 |
1.03,
s |
- |
|
29 |
109.35
CH2 |
4.57,
dd |
3.9,
4.2 |
|
30 |
55.37
CH3 |
0.79,
s |
- |
|
1’ |
O |
- |
- |
|
2’ |
171.02
C |
- |
- |
|
3’ |
57.53CH3 |
0.78,
s |
- |
Compound
Ea-7-38 was obtained as white solid which appeared green under UV light,
Reddish brown when sprayed with MPA and has a melting point of 368oC
and Rf value of 0.42 in a hexane/DCM 9:1 solvent ratio. Compound Ea-9-10 was
also obtained as white solid which appeared green under UV light, Reddish brown
when sprayed with MPA and has a melting point of 694oC and Rf value
of 0.51 in a hexane/DCM 8:2 solvent ratio. Compound Ea-12-85 was obtained as
white solid which appeared green under UV light, brown when sprayed with MPA
and has a melting point of 521oC and Rf value of 0.50 in a
hexane/DCM 7:3 solvent ratio (Table
2). Compound Ea-7-38 was obtained as white
solid with molecular formula C32H52O2 from
HR-ESIMS m/z 491.2273 [M+Na]+ (calculated
for C32H52O2Na 491.2273.
Table 4. 13C and 1H NMR
data (Chloroform-d1 700 MHz for 1H and 176 MHz 13C) for
Compound Ea-9-10
|
C Position |
ẟC
Ea-9-10 isolated |
ẟH/
Multiplicity |
J value (Hz) |
|
1 |
38.85 CH2 |
1.007d |
13.0 |
|
2 |
27.39 CH2 |
1.645, q |
13.3 |
|
3 |
80.98 CH |
3.229 dd |
11.5,5.8 |
|
4 |
38.83 C |
- |
- |
|
5 |
55.33 CH |
0.739, ddd |
10.9,10.0,9.9 |
|
6 |
18.41 CH2 |
1.544 q |
13.0 |
|
7 |
34.20 CH2 |
1.417, t |
13.5 |
|
8 |
41.03 C |
- |
- |
|
9 |
49.80 CH |
1.446 t |
13.1 |
|
10 |
37.22 C |
- |
- |
|
11 |
22.23 CH2 |
1.523, q |
4.2 |
|
12 |
24.92 CH2 |
1.885 q |
5.8 |
|
13 |
135.41 C |
- |
- |
|
14 |
44.98C |
- |
- |
|
15 |
26.39CH2 |
1.046 q |
3.4 |
|
16 |
38.89 CH2 |
1.459, d |
14.5 |
|
17 |
34.30C |
- |
- |
|
18 |
136.38C |
- |
- |
|
19 |
38.28 CH |
2.61, q |
7.5 |
|
20 |
34.97CH |
1.679s |
6.9 |
|
21 |
23.71 CH2 |
1.131, q |
13.7 |
|
22 |
36.32 CH2 |
1.38, t |
13.2 |
|
23 |
28.28 CH3 |
0.99 s |
|
|
24 |
15.99 CH3 |
0.77, s |
|
|
25 |
16.49 CH3 |
0.87 s |
|
|
26 |
17.35 CH3 |
0.92 s |
|
|
27 |
21.17 CH3 |
1.12s |
|
|
28 |
28.27 CH3 |
1.10 s |
|
|
29 |
23.15 CH2 |
1.09, dd |
7.6,7.0 |
\
Table 5. 13C and 1H NMR data (Chloroform-d1
700 MHz for 1H and 176 MHz for 13C) for Compound Ea-12-85
|
C Position |
ᵟC Ea-12-85 Isolated Cpd |
ᵟH/ Multiplicity |
J value (Hz) |
|
1 |
38.85 CH2 |
1.007d |
13.0 |
|
2 |
27.39 CH2 |
1.645, q |
13.3 |
|
3 |
80.98 CH |
3.229 dd |
11.5,5.8 |
|
4 |
38.83 C |
- |
- |
|
5 |
55.33 CH |
0.739, ddd |
11.9,10.5,9.9 |
|
6 |
18.41 CH2 |
1.544 q |
13.0 |
|
7 |
34.20 CH2 |
1.417, t |
13.5 |
|
8 |
41.03 C |
- |
- |
|
9 |
49.80 CH |
1.446 t |
13.1 |
|
10 |
37.22 C |
- |
- |
|
11 |
22.23 CH2 |
1.523, q |
4.2 |
|
12 |
24.92 CH2 |
1.885 q |
5.8 |
|
13 |
135.41 C |
- |
- |
|
14 |
44.98C |
- |
- |
|
15 |
26.39CH2 |
1.046 q |
3.4 |
|
16 |
38.89 CH2 |
1.459, d |
14.5 |
|
17 |
34.30C |
- |
- |
|
18 |
136.38C |
- |
- |
|
19 |
38.28 CH |
2.61, q |
7.5 |
|
20 |
34.97CH |
1.679 |
6.9 |
|
21 |
23.71 CH2 |
1.131, q |
13.7 |
|
22 |
36.32 CH2 |
1.38, t |
13.2, |
|
23 |
28.28 CH3 |
0.99 s |
0.4 |
|
24 |
15.99 CH3 |
0.77, s |
0.4 |
|
25 |
16.49 CH3 |
0.87 s |
1.0 |
|
26 |
17.35 CH3 |
0.92 s |
0.6 |
|
27 |
21.17 CH3 |
1.12s |
1.0 |
|
28 |
28.27 CH3 |
1.10 s |
0.7 |
|
29 |
23.15 CH2 |
1.09, dd |
7.6,8.0 |
|
30 |
179.8 CO |
- |
- |
|
|
OH |
1.26 |
6.0 |
|
|
OH |
1.26 |
- |
The proton (1H) NMR, 13C NMR (Table 3) and
IR suggested that Ea-7-38 might be a triterpene with the cluster of methylene
and methyl protons at δ 2.0 – 0.1 values range. A
terminal C=CH2 protons were conspicuous at δ value 4.69
and 4.68, a δ value of 4.57 corresponds to
O-CH protons. A δ
value of 2.3 corresponds to C=C-CH3 protons, a singlet signal of
high intensity at δ value of 2.0 reveal the OCH3 protons. The carbon-13
NMR revealed a 32-carbon compound with a carbonyl carbon at 171ppm, two peaks
at δ150ppm and δ109ppm
corresponding to H2C=CR2, a moderate peak at δ81ppm
revealed an O-CH ring carbon. The peak at δ77ppm with high intensity is
the solvent (deuterated chloroform). The 1D carbon-13 NMR, 2D DEPT 90, 45 and
135 (Supplementary
Fig S1-S10) revealed 11 CH2, 8 CH3, and 6 CH groups, IR
spectra revealed a carbonyl (C=O) absorption band at 1732.629cm-1
C-O stretch at 1245cm-1 indicating an ester, C-H stretch (broad) at
2942.273cm-1, C=C-H stretch at 3074.489cm-1 typical of
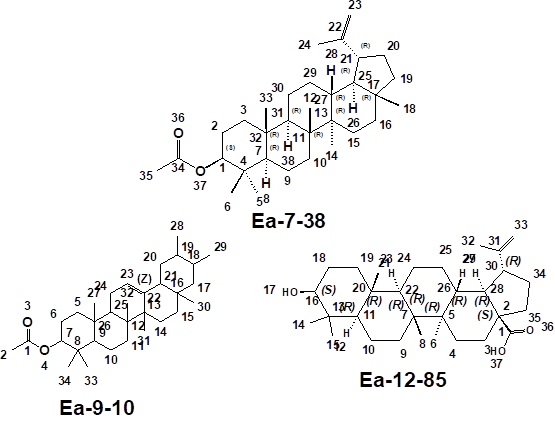
esters. From the relevant literature, compound Ea-7-38 was identified as a pentacyclic triterpene
3a,5,5b,8,8,11a-hexamethyl-1-(prop-1-en-2-yl)
icosahydro-1H-cyclopenta[a]chrysene -9-yl acetate (Lupeol acetate).
Compound Ea-9-10 was obtained as white
solid with molecular formula C32H52O2 from
HR-ESIMS m/z 390.2273 [M+Na]+ (calculated for C32H52O2Na
390.2273). The proton (1H) NMR, 13C NMR (Table 4), and IR
suggested that Ea-7-38 is a triterpene with the cluster of methylene and methyl
protons at δ 2.0 – 0.5 values range. A ring (internal alkene) HC=CH protons
were conspicuous at δ value 4.69 and 4.68, a δ value of 4.57 corresponds
to O-CH protons. A δ value of 2.3 corresponds to
>C=C-CH3 protons, a singlet signal of high intensity at δ value of
2.0 reveal the OCH3 protons. The carbon-13 NMR revealed a 32-carbon
atoms compound with a carbonyl carbon at δ171ppm, two peaks at δ144ppm and δ139ppm
corresponding to C=C isolated ring alkene, a moderate peak at δ81ppm
revealed a O-CH ring carbon. The peak at δ77ppm with high intensity is
the solvent (deuterated chloroform). The 1D carbon-13 NMR, 2D DEPT 90, 45 and
135 revealed 11 CH2, 8CH3, and 6CH groups. The IR
spectrum revealed a carbonyl (C=O) absorption band at 1732.629cm-1,
C-O stretch at 1245cm-1, C-H stretch (broad) at 2942.273cm-1,
>C=C-H stretch at
3074.489cm-1 typical of an ester. Table 4 provided the data (carbons
position, 1H multiplicity, coupling constant, 1H-1H
COSY) for Ea-9-10 while Table 4. 24 avails the comparative data from standard
(MestReNova), Literature and isolated compound Ea-9-10. From these data and the
library data (Table 6) obtained from both ChemBioDraw Ultra 3.0 and MestReNova application software, compound Ea-9-10 was
identified as a triterpene 4,4,6a,6b,8a,10,11,14b, octamethyl
1,1,2,3,4,4a,5,6,6a,6b, 7,8,8a,9,10,11,12,12a, 14,14a,14b icosahydropicen- 3-yl
acetate (derivative of β-Amyrin).
Table 6. BST Assay Results of Compound 1
|
Conc. (µg/mL) |
Survivals |
Deaths |
Mortality
(%) |
Log10 Conc. |
|||||
|
|
V1 |
V2 |
V3 |
V1 |
V2 |
V3 |
|
|
LC50(µg/mL) 1336.5 |
|
1000 |
10 |
9 |
9 |
0 |
1 |
1 |
6.67 |
3 |
|
|
500 |
9 |
10 |
8 |
1 |
0 |
2 |
10 |
2.7 |
|
|
250 |
8 |
10 |
9 |
2 |
0 |
1 |
10 |
2.4 |
|
|
125 |
9 |
9 |
9 |
1 |
1 |
1 |
10 |
2.1 |
|
|
62.5 |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
1.8 |
|
|
Ctrl(+) |
0 |
0 |
0 |
10 |
10 |
10 |
100 |
|
|
|
Ctrl(-) |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
|
|
Table 7: BST Assay Results of Compound 2
|
Conc. (µg/mL) |
Survivals |
Deaths |
Mortality
(%) |
Log10 Conc |
|
||||
|
|
V1 |
V2 |
V3 |
V1 |
V2 |
V3 |
|
|
LC50(µg/mL) 1036.5 |
|
1000 |
8 |
9 |
7 |
2 |
1 |
3 |
20.0 |
3 |
|
|
500 |
9 |
10 |
8 |
1 |
0 |
2 |
10 |
2.7 |
|
|
250 |
8 |
9 |
8 |
2 |
1 |
1 |
16.67 |
2.4 |
|
|
125 |
9 |
10 |
9 |
1 |
0 |
1 |
6.67 |
2.1 |
|
|
62.5 |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
1.8 |
|
|
Ctrl(+) |
0 |
0 |
0 |
10 |
10 |
10 |
100 |
|
|
|
Ctrl(-) |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
|
|
Table 8. BST Assay Results of Compound 3
|
Conc. (µg/mL) |
Survivals |
Deaths |
Mortality
(%) |
Log10 Conc |
LC50(µg/mL) 136.5 |
||||
|
|
V1 |
V2 |
V3 |
V1 |
V2 |
V3 |
|
|
|
|
1000 |
3 |
2 |
2 |
7 |
8 |
8 |
76.67 |
3 |
|
|
500 |
5 |
1 |
8 |
5 |
9 |
2 |
53.33 |
2.7 |
|
|
250 |
8 |
7 |
8 |
2 |
3 |
2 |
26.67 |
2.4 |
|
|
125 |
9 |
10 |
6 |
1 |
0 |
4 |
16.67 |
2.1 |
|
|
62.5 |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
1.8 |
|
|
Ctrl(+) |
0 |
0 |
0 |
10 |
10 |
10 |
100 |
|
|
|
Ctrl(-) |
10 |
10 |
10 |
0 |
0 |
0 |
0.00 |
|
|
Compound Ea-12-85 was obtained as white
solid and molecular formula proposed to be C30H49O from
HR-ESIMS m/z 390.2273 [M+Na]+(calculated for C30H49ONa,
337). The proton (1H) NMR, 13C NMR (Table 5) and IR
suggest that Ea-12-85 might be a triterpene with the cluster of methylene and
methyl protons at δ 2.0 – 0.1 values range. A ring (internal alkene) HC=CH protons
were conspicuous at δ value 4.69 and 4.68, a δ value of 4.57 corresponds
to O-CH protons. A δ value of 2.3 corresponds to C=C-CH3 protons, a singlet signal of
high intensity at δ value of 2.0 reveal the OCH3 protons. The carbon-13
NMR revealed a 32-carbon compound with a carbonyl carbon (acid) at δ179ppm, two
peaks at δ144ppm and δ139ppm corresponding to C=C isolated ring alkene, a moderate peak
at 81ppm revealed a O-CH ring carbon. The peak at δ77ppm with
high intensity is typical of the solvent (deuterated chloroform). The 1D
carbon-13, 2D DEPT90, 45 and 135 revealed 11 CH2, 8 CH3,
and 6 CH groups. The IR spectrum revealed a carbonyl (C=O) absorption band at
1732.629cm-1, C-O stretch at 1245cm-1 revealing an ester,
O-H stretch (broad) at 3242.273cm-1 revealing an alcohol, C=C-H
stretch at 3074.489cm-1 indicating an alkene. From the relevant
literature compound Ea-12-85 was identified as betulinic acid. The earlier work
reported by Oliver et al., 2015 on the isolation of triterpene from
plants was in agreement and supported this submission with comparative data.
3.2 Brine Shrimp Lethality Test
The result of the in vitro
cytotoxicity studies (Tables 6 - 8) revealed that Compounds 1 and 2 (Fig. 1)
were non-toxic (LC50(µg/mL) ≥1000) to the
nauplii while compound 3 (Fig. 1) was toxic (LC50(µg/mL) = 136.5) to the nauplii. In similar studies terpenes have been reported to exert anti-inflammatory effects by
inhibiting various proinflammatory pathways in ear edema, bronchitis, chronic
obstructive pulmonary disease, skin inflammation, and osteoarthritis [12-15, 16]. A natural compound linalool
found in essential oils of aromatic plants, inhibited cigarette smoke-induced
acute lung inflammation [17]. The
findings of this study suggest that the plant extracts are reliable natural
sources of terpenes terpenoids and their derivatives. A report revealed that structurally related monoterpenes p-Cymene,
carvacrol and thymol isolated from essential oil from leaves of Lippia
sidoides cham. (Verbenaceae) protected mice against elastase-induced
emphysema [18]. Also based on the reported antiplasmodial
studies carried out on solvent extracts of this plant [19], the use of this plant by traditional
medicine practitioners especially at lower doses to cure jaundice and malaria
becomes scientifically valid especially in rural communities where orthodox
drugs are unaffordable because of the costs.
The three isolated compounds were found to be nontoxic to the
shrimps except one. However, the study revealed that the stem bark extract of
this plant contained important secondary metabolites which include but not
limited to triterpenes and their derivatives. A report by [12] revealed that pentacyclic
lupane-type triterpenes, possess beneficial effects as a therapeutic and
preventive agent for a range of disorders which include anti-inflammatory and
anti-arthritic activities both in in vitro and in vivo systems.
There has been a tremendous effort by researchers worldwide to develop the use
of triterpenes toward the treatment of a variety of disorders especially the
mechanism of action of lupeol and suggest that it is a multi-target agent with
immense anti-inflammatory potential targeting key molecular pathways which
involve nuclear factor kappa B, phosphatidylinositol-3-kinase in a variety of
cells. It was reported that lupeol at its effective therapeutic doses exhibits
no toxicity to normal cells and tissues. The efficacy of triterpenes as
antitumor (carcinogenesis) by the use of either natural or synthetic substances
individually or in combination therapy was promising. However, there was no report
on the use of triterpenes as remedy to malaria disease.
Figure 1. 1D and 3D
structures of the Isolated Compounds
4. Conclusions
Our
current study revealed that dichloromethane
and methanol extracts of Echinaceae angusifolia DC (Compositae) contained significant amount of
some secondary metabolites with the methanol extracts indicating the presence
of most of the secondary metabolites. The Secondary metabolites present
includes flavonoids, terpenes, saponins, steroids, alkaloids and tannins. The
revealed cytotoxicity activity in this study is due to the triterpenes isolated
from the plant extract. Consequently, this scientific information can
serve as an important baseline data for the development of safe and effective
natural medicine.
Supplementary Data
DOI Link:
https://doi.org/10.58985/jeopc.2023.v01i02.18
Authors’
contributions
AZ: conducted the research work, SJ: assisted in
chromatography and spectral analysis, FMO: Supervision of the research work and
helped for the structural elucidation, MHS: assisted in the purification and
TLC experiment, AS: assisted in VLC and general extractions.
Acknowledgements
The
authors wish to acknowledge the Tertiary Education Trust Fund (TETFund –
Nigeria) for the research grant. The government of the Sultanate of Oman for
granting travel VISA and Sultan Qaboos University for the Bench space to carry
out the research. The tireless effort of the staff of Central Analytical and
Applied Research Unit (CAARU) Sultan Qaboos University Oman in carrying out the
MS and NMR analysis is appreciated.
Funding
The Tertiary Education Trust Fund (TETFUND) Nigeria
funded the Three (3) years PhD program and a one-year oversea Bench-Work via
FUK/R/SS/SF/184/100 and FUK/R/SS/SF/184/101 respectively.
Conflicts of interest
The
Authors wish to declare that there is no conflict of interest.
References
1.
Nardos,
A.; Makonnen, E. In vivo antiplasmodial activity and toxicological
assessment of hydroethanolic crude extract of Ajuga remota Mal.
J. 2017, 16, 25.
2.
Callies,
O.; Bedoya, L. M.; Beltran, M.; Munoz, A.; Calderón, P. O.; Osorio, A. A.;
Bazzocchi, I. L. Isolation, structural modification, and HIV inhibition of
pentacyclic lupane-type triterpenoids from Cassine xylocarpa and Maytenus
cuzcoina. J. Nat. Prod.
2015, 78(5), 1045-1055.
3.
Nyalambisa,
M.; Oyemitan, I. A.; Matewu, R.; Oyedeji, O.O.; Oluwafemi, O.S.; Songca, S.P.;
Oyedeji, A.O. Volatile constituents and biological activities of the leaf and
root of Echinacea species from South Africa. Saudi Pharm. J.
2017, 25(3), 381-386.
4.
Spelman, K.; Iiams-Hauser,
K.; Cech, N.B.; Taylor, W.; Smirnoff, N.; Wenner, C.A., Role
for PPARγ in IL-2 inhibition in T cells by Echinacea-derived
undeca-2E-ene-8,10-diynoic acid isobutylamide
Int. Immunopharmacol. 2009, 9, 1260–1264.
5.
Farinacci, M.; Colitti, M.; Stefanon, B.
Modulation of ovine neutrophil function and apoptosis by standardized extracts
of Echinacea angustifolia, Butea frondosa and Curcuma longa. Vet. Immun. Immunopathol. 2009,
128, 366–373.
6.
Cucuzza, S.; Motta, M.; Accornero, P.;
Baratta, M. Effect of Echinacea augustifolia extract
on cell viability and differentiation in mammary epithelial cells.
Phytomedicine. 2008, 15, 555–562.
7.
Wu, H.; Nardone, A.; Lacetera N. Effects
of a standardized purified dry extract from Echinacea angustifolia on
proliferation and interferon gamma secretion of peripheral blood mononuclear
cells in dairy heifers. Vet. Sci.
2009, 87, 396–398.
8.
Fusco, D.; Liu, X.; Savage, C.; Taur,
Y.; Xiao, W.; Kennelly, E.; Yuan, J.; Cassileth, B.; Salvatore, M.; Genovefa,
A. Papanicolaou. Echinacea purpurea aerial
extract alters course of influenza infection in mice Vaccine. 2010, 28,
3956–3962.
9.
Wal, P.; Wal, A.; Sharma,
G.; Rai, A.K. Biological activities of
lupeol, Sys. Rev. Pharm. 2011,
2 (2).
10.
Callies, O.; Bedoya, L.M.; Beltran, M.;
Munoz, A.; Calderon, P.O.; Osorio, A.A.; Jimenez, I.A.; Alcami J.; Bazzocchi,
I.L. Isolation, structural modification,
and HIV inhibition of pentacyclic lupane-type triterpenoids from Cassine
xylocarpa and Maytenus cuzcoina. J. Nat. Prod. 2015, 78, 1045-1055.
11.
Adoum O.A. Insecticidal activity of some
savannah plants. PhD thesis from Bayero University Kano (unpublished), 2000.
12.
See, D.;
Berman, S.; Justis, J.; Broumand, N.; Chou, S.; Chang, J.; Tilles, J. A phase I study on the safety of Echinacea
angustifolia and its effect on viral load in HIV infected individuals. J. Am. Nutr. Assoc., 1998, 1, 14-17.
13.
Khiev,
P.; Kwon, O.K.; Song, H.H.; Oh, S.R.; Ahn, K.S.; Lee, H.K.; Chin, Y.W.
Cytotoxic terpenes from the stems of Dipterocarpus
obtusifolius collected in Cambodia. Chem. Pharm. Bul. 2012, 60(8), 955-961.
14.
Games, E.; Guerreiro, M.; Santana, F.R.;
Pinheiro, N.M.; de Oliveira, E.A.; Lopes, F.D.; Olivo, C.R.; Tibério, I.F;
Martins, M.A; Lago, J.H.; Prado, C.M. Structurally related monoterpenes
p-cymene, carvacrol and thymol isolated from essential oil from leaves of Lippia sidoides Cham. (Verbenaceae)
protect mice against elastase-induced emphysema. Molecules 2016, 21(10), 1390.
15.
Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.;
Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of
(+)-α-pinene: structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269.
16.
Ma, J.;
Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool inhibits cigarette
smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol., 2015, 29(2), 708-713.
17.
Rodrigues,
F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F. C.; Carneiro, S.M.P.; de Amorim,
C.F.A. Syzygium cumini (L.) Skeels
essential oil and its major constituent α-pinene exhibit anti-leishmania
activity through immunomodulation in vitro. J. Ethnopharmacol, 2015,
160, 32-40.
18.
Li, X.J.;
Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and 1-octanol
contribute to the topical anti-inflammatory and analgesic activities of
frankincense by inhibiting COX-2. J. Ethnopharmacol, 2016, 179, 22-26.
19.
Yu, P.J.;
Wan, L.M.; Wan, S.H.; Chen, W.Y.; Xie, H.; Meng, D.M.; Xiao, X.L. Standardized
myrtol attenuates lipopolysaccharide induced acute lung injury in mice. Pharm.
Biol, 2016, 54(12),
3211-3216.
20.
Usman,
H.; Abdulrahman, F.I.; Usman, A. Qualitative phytochemical screening and in
vitro antimicrobial effects of methanol stem bark extract of Ficus thonningii (Moraceae). Afr. J. Trad. Complemen. Altr. Med. 2009,
6(3).
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
From the Stems
of Bark of Echinaceae angustifolia DC
three known triterpenes
3a,5,5b,8,8,11a-hexamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysene-9-yl
acetate (lupeol acetate),
4,4,6a,6b,8a,10,11,14b,octamethyl1,1,2,3,4,4a,5,6,6a,6b,7,8,8a, 9,10,
11,12,12a,14,14a,14b-icosahydropicen-3-yl acetate (derivative of β-amyrin and
9-hydroxy-1-isopropenyl-5a,5b,8,8,11a-pentamethyl-icosahydro-cyclopenta[a]chrysene-3a-carboxylic
acid (betulinic acid), labelled as Ea-7-38, Ea-9-10 and Ea-12-85) were isolated
and characterized. All isolates were tested for their cytotoxicities against Artemia salina (brine shrimp larvae).
Compound Ea-12-85 exhibited potent cytotoxic activity against the Artemia salina, Ea-7-38, Ea-9-10 were
found to be non-toxic in the cytotoxicity test. The result of the study has
justified the claim of the traditional medicine practitioners in Girei for the
treatment of complicated malaria disease using the stem bark of E. angustifolia DC.
Abstract Keywords
Isolation, 2D NMR,
structural elucidation, triterpene, ethnomedicinal, Echinaceae
angustifolia DC
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


