Research Article
Edache Joseph
Edache Joseph
Department
of Pharmaceutical Services, University of Maiduguri Teaching Hospital,
Maiduguri, Borno State, Nigeria.
Yesufu Braimah Hassan⁎
Yesufu Braimah Hassan⁎
Corresponding Author
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Maiduguri, Maiduguri, Borno State, Nigeria.
E-mail: hbyesufu@unimaid.edu.ng, Tel: +2348054035629
Abdulqadir Bukar Bababe
Abdulqadir Bukar Bababe
Department
of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Maiduguri,
Maiduguri, Borno State, Nigeria.
Garba Mohammed Tom
Garba Mohammed Tom
Department
of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Maiduguri,
Maiduguri, Borno State, Nigeria.
Received: 2024-12-28 | Revised:2025-02-26 | Accepted: 2025-02-28 | Published: 2025-03-14
Pages: 46-54
DOI: https://doi.org/10.58985/jpam.2025.v03i02.31
Abstract
This
study took a closer look at the leaves of Momordica balsamina, a plant
long valued in African folk medicine. The leaves were extracted using methanol
and a gentle cold maceration method. Then, it was analyzed for its chemical
composition and carefully separated into different fractions: n-hexane,
chloroform, and n-butanol. The extracts were tested against common pathogens
like E. coli,
S. pyogenes,
P. aeruginosa,
and C. albicans.
The results were found to be promising, with better inhibition of E. coli
and S. pyogenes
at 12.5 μg/mL present in the n-hexane fraction.
The MBC result shows the crude extract and the n-hexane fraction being
bactericidal against E. coli and S. pyogenes at 1.25
μg/mL and 5 μg/mL, the
phytochemical analysis revealed a variety of beneficial compounds, including
carbohydrates, steroids, and flavonoids, while noting that anthraquinone was
absent. The extract and its n-hexane fraction showed effectiveness against
E. coli
and S.
pyogenes, but had only a minimal impact on P. aeruginosa
and no significant antifungal effects. Interestingly, the
research led to the isolation of two compounds—dodec-1-ane (C10H21
CH=CH2) and trilinolein glyceride (C57H98O6)—for
the first time from M.
balsamina leaves.
Overall, these findings reinforce the plant's traditional use in treating
infections and suggest it could be a valuable source for developing new
antimicrobial treatments.
Keywords
M. balsamina leaves, cold maceration, pathogens, phytochemicals, dodec-1-ene, trilinolein glyceride.
1. Introduction
The increasing prevalence of antimicrobial resistance and the high cost of available antibiotics have necessitated the search for new and cost-effective alternatives for the treatment of infectious diseases [1-2]. The World Health Organization (WHO) has reported that antimicrobial resistance is a major public health concern, with the potential to undermine the effectiveness of modern medicine [3]. In this context, medicinal plants have been a rich source of antimicrobial compounds, and their use in traditional medicine has been widely documented [4-5].
The use of medicinal plants dates back thousands of years, with evidence of their use found in ancient civilizations such as Egypt, China, and Greece [6-7]. The Genus Mormodica consists of 47 species in Africa and 12 in Asia and Australia. They are mainly annual or perennial climbers. Some species within this genus include M. charantia, M. balsamina, M. cymbalaria, M. Subangulata, M. sahyadrica [8]. The use of traditional medicine, including herbal remedies, has experienced a resurgence in recent years due to the increasing side effects of orthodox medicines, resistance cases of antibiotics, and lack of curative treatments for several chronic diseases [9]. Herbal medicines have been used to manage mild to complicated ailments, including wound healing, bleeding, miscarriages, toothaches, and the extraction of bullets from gunshots [10].
Momordica balsamina, a plant belonging to the family Cucurbitaceae, has been traditionally used in the treatment of various infectious conditions, including bacterial, viral, and fungal infections [11-12]. Previous studies have reported the presence of various phytochemicals, including alkaloids, flavonoids, and phenolic acids, in M. balsamina [13-14]. These phytochemicals have been reported to possess antimicrobial, anti-inflammatory, and antioxidant activities [15]. The major plant-derived chemical groups in this family now recognized as having potential health promoting effects in Type I and Type II diabetes are cucurbitane triterpenoids, saponin glycosides and Momordica anti-HIV protein (MAP 30 protein). However, with growing resistance to the available anti-microbial agents, more studies to uncover better and more efficient drugs that can curb the ailing effect due to microorganisms are required.
2. Materials and methods
2.1. Plant collection
and identification
Mormodica balsamina leaves were collected within the University of Maiduguri Staff Quarters, Maiduguri Metropolis, Borno State, Nigeria in the month of August, 2016. The plant material was identified and authenticated by a taxonomist in the Department of Pharmacognosy, University of Maiduguri. A voucher specimen was deposited in the Herbarium of the Department of Pharmacognosy, University of Maiduguri and a voucher number UMM/FPH/CUB/001 was allotted.
2.2 . Test organisms
The test organisms used for this study were Streptococcus pyogenes (ATCC 19615), Pseudomonas aeruginosa (ATCC 9027), Escherichia coli (ATCC 43888) and Candida albicans (ATCC 10231). They were obtained from the Microbiology Laboratory of the National Veterinary Research Institute (NVRI) Vom, Jos, Plateau State, Nigeria. The organisms were kept at 4 OC prior to bioassay of the extracts.
2.3 . Plant extraction
The leaves of Momordica balsamina were cleaned and air-dried under shade at normal room temperature and then pulverized into coarse powder using mortar and pestle. The air-dried powdered sample of 1kg was macerated by soaking in 3 liters of 100% methanol for 72 hours. The crude extract obtained was subjected to filtration and the filtrate was concentrated by air-drying at normal room temperature and the concentrate was labeled. The yield (% w/w) of the concentrate was then calculated and subjected to various chemical tests.
2.4.
Phytochemical screening
The crude methanol extract was subjected to qualitative analysis to identify the various classes of bioactive chemical constituents such as steroids, tannins, flavonoids, alkaloids, terpenes, saponins, saponin glycosides, cardiac glycosides etc. according to the methods [16-17].
2.5.
Fractionation of crude extract
The crude extract (50g) was suspended in hot water and filtered. The resulting filtrate was then subjected to partitioning successively using the following solvents in order of increasing polarity: n-hexane (500 mL x3), chloroform (500 mL x3) and n-butanol (500 mL x3). The resultant fractions were evaporated to dryness and kept in airtight container for further analysis.
2.6.
Antimicrobial investigation
The crude extract and the various fractions were subjected to antimicrobial assay using standard methods of agar disc diffusion method, MIC, MBC and MFC studies. The microorganisms were sub-cultured in nutrient broth at 37 oC for 24 h prior to the antimicrobial screening.
The disc diffusion agar method (Kirby-Bauer test) was used to test for the susceptibility of the microorganisms to the extract on Mueller-Hinton agar. The prepared agar plate was first spread with the microorganism and paper disc of the negative control, positive controls and varying concentrations of the extract and fractions corresponding to 10, 5 and 2.5 mg were added. The microorganism was then allowed to grow and the zones of inhibition around each disc was measured. The negative control was prepared using water containing three drops of tween-80 while the positive controls were 10 µL of ciprofloxacin disc and 10 µL ketoconazole disc for bacterial and fungal strains respectively.
Various concentrations of the crude extract and fractions were inoculated with cultured microorganisms, and the results were measured using the broth dilution method to determine at what level of the MIC endpoint was established. The varying concentrations were added to nutrient broth in a tube together with the negative standard and the standard antibiotic. These were then incubated and checked for the presence of growth. The lowest concentration (highest dilution) of the fractions that produced no visible growth (no turbidity) when compared with the control tubes that were considered as the initial MIC. The dilutions that showed no turbidity were incubated further for 24 h at 37 °C. The lowest concentration that produced no visible turbidity after a total incubation period of 48 h was regarded as the final MIC [18].
The
Minimum Bactericidal (Fungicidal) Concentration (MBC/MFC) is the lowest
concentration of an antibacterial agent required to kill a bacterium (fungus)
over a fixed, somewhat extended period, such as 18 hours or 24 hours, under a
specific set of conditions. It was
determined from the broth dilution of MIC tests by subculturing to agar plates
that do not contain the test agent. The MBC/MFC was identified by determining
the lowest concentration of antibacterial agent that reduces the viability of
the initial bacterial inoculum by a pre-determined reduction such as ≥99.9% [18].
2.7.
Isolation of bioactive principles
The n-hexane fraction (20 g) of M. balsamina leaves was subjected to chromatographic separation using an open glass column (48 cm long and 4.5 cm in diameter). The column was packed wet with silica gel as the stationary phase. Elution was carried out with solvents of increasing polarity, starting with 100% n-hexane and progressing to chloroform, chloroform/methanol, and methanol [19-20].
3. Results and discussion
3.1. Preliminary phytochemical screening
The preliminary phytochemical screening revealed the presence of carbohydrates, steroids and triterpenes, saponins, tannins, and flavonoids and anthraquinone glycosides, while cardiac glycosides were absent (Table.1).
Table 1. Preliminary Phytochemical Screening of the Methanol Extract of M. balsamina leaves.
Phytochemical constituents | Tests | M | C | H |
Carbohydrates | Molisch’s Test | + | + | + |
Reducing sugars | + | + | + | |
Combined reducing sugars | + | + | + | |
Test for monosaccharides | + | + | + | |
Tannins | Ferric Chloride | + | + | + |
Lead acetate | + | + | + | |
Anthraquinones | Bontrager’s Test | - | - | - |
Free | - | - | - | |
Combined | - | - | - | |
Saponins | Frothing test | + | + | + |
Haemolysis test | + | + | + | |
Cardiac glycosides | Killer Kiliani Test | + | + | + |
Liberman Burchard Test | - | - | + | |
Salkowski Test | - | - | - | |
Cyanogenic glycoside |
|
|
|
|
Flavonoids | Lead Acetate test | + | + | + |
Sodium Hydroxide test | - | - | - | |
Alkaloids | Mayers Test | + | + | + |
Dragendorff’s Test | + | + | + | |
H= n-hexane, C= Chloroform M-methanol | ||||
The phytochemical screening result for the leaves of M. balsamina shows the presence of carbohydrates, tannins, saponins, cardiac glycosides, flavonoids and alkaloids. Similar results were obtained by Jaichand et. al. [21] which showed the presence of alkaloids, saponins, cardiac glycosides, steroids and triterpenoids. However, the study by Jaichand et. al. [21], did not show the presence of tannins. Osuntokun and Ajayi [22] in their study, demonstrated the presence of alkaloids, saponins, cardiac glycosides, steroids, phenols, tannins, flavonoids and philobatain. Thakur et. al., [23] revealed the presence of tannins in addition to the other phytochemicals above. The observed difference may be as a result of differences in the mode of extraction, climatic, geographic and environmental conditions and the age of the plant used. The presence of these phytochemicals is responsible for the many biological effects of this plant.
3.2. Antimicrobial studies
The results from the antimicrobial tests suggest that the leaves of M. balsamina possess dose dependent antimicrobial properties (Table 2). This is in agreement with the results obtained by Ramalhete et. al. [24], Bello et. al. [12] and Nogbou et. al., [25], all of which suggest the presence of antimicrobial properties of various parts of M. balsamina. The agar diffusion test showed zones of inhibition that were highest in S. Pyogenes among the bacteria. This could be attributed to the fact that it is a Gram-positive bacterium while the others are Gram-negative. Koohsari et. al. [26] reported that gram-positive bacteria are more susceptible to antimicrobial agents than gram-negative bacteria.
Table 2: Zones of inhibition (mm) at varying concentrations (mg/ mL) of the fractions
Treatment groups |
Conc. | Zones of inhibition (mm) | |||
E. coli | S. pyogenes | P. aeruginosa | C. albicans | ||
Crude Extract | 10 mg | 13.33 ± 0.57 | 15.33± 1.53 | 0.0 ± 0.00 | 8.67± 1.57 |
| 5 mg | 10.53 ± 1.53 | 11.33 ± 1.15 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 2.5 mg | 10.00 ±1.73 | 11.0 ± 1.73 | 0.0 ± 0.00 | 0.0 ± 0.00 |
n-Hexane | 10 mg | 13.33 ± 1.15 | 13.33± 1.15 | 8.67 ± 1.57 | 0.0 ± 0.00 |
| 5 mg | 12.67 ± 2.31 | 12.67 ± 1.15 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 2.5 mg | 12.00± 1.00 | 12.67 ± 1.15 | 0.0 ± 0.00 | 0.0 ± 0.00 |
Chloroform | 10 mg | 7.67± 0.58 | 9.00 ± 1.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 5 mg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 2.5 mg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
n-butanol | 10 mg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 5 mg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 2.5mg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
Std. 1 | 10μg | 35.0 ± 0.00 | 40.0 ± 0.00 | 25.0 ± 0.00 | 0.0 ± 0.00 |
Std. 2 | 10 μg | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 23.0 ± 0.00 |
Negative |
| 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
Std. 1 =Std. (ciprofloxacin); STD. 2= Std. 2 (Ketoconazole) negative= Negative control (Distilled water + 2 drops of tween-80); 0 = No activity | |||||
Among the various fractions, the n-hexane fraction, a non-polar fraction, displayed a more potent antibacterial effect when compared to the others. This is in contrast to the findings of Abdulhamid et. al. [27] which show that the methanol and n-butanol (polar) fractions have more potent antibacterial effect than the non-polar fractions. This contrast could possibly be as a result of the variation of the constituent of the fractions that were able to inhibit the growth of the bacteria, the geographical differences where the plants were collected and the different types of bacteria involved in the various studies could also be a factor.
The MIC is a measure of the bacteriostatic activity, which indicates an agent inhibits the growth of cells but does not kill them [28]. The observed MIC against E. coli was 2.5, 1.25, and 10 mg/mL, respectively, for the crude, n-hexane and chloroform fractions 9 (Table 3).
Table 3. Minimum inhibitory concentration (MIC) of the various fractions (μg/mL)
Organisms | Crude extract | n-Hexane | Chloroform | n-Butanol |
E. coli | 2.5 | 1.25 | 10 | N/C |
S .pyogenes | 1.25 | 1.25 | 10 | 5 |
P. aeruginosa | 10 | TNC | TNC | TNC |
C. albicans | TNC | TNC | TNC | TNC |
TNC- Test Not Conducted | ||||
This suggests a greater bacteriostatic activity against E. coli with the n-hexane fraction when compared to the others. There was no MIC recorded with the n-butanol fraction. For the S. pyogenes, the lowest MIC values were recorded with the crude extract and the n-hexane fractions, also suggesting that the non-polar fractions have more antibacterial effects. Pseudomonas aeruginosa showed more resistance, having shown MIC values for just the crude extract, which was the highest observed MIC value.
The MBC result shows the crude extract and the n-hexane fraction being bactericidal against E. coli and S. pyogenes (Table 4). The study recorded no antifungal activity. This result is in agreement with the study conducted by Otimenyi et. al. [29] which suggests that M. balsamina has no inhibitory effect on various fungal species.
Table 4. Minimum Bactericidal concentration (MBC) of the various fractions (μg/mL)
Orgnisms | Crude extract | n-Hexane | Chloroform | n-Butanol |
E. coli | 5.0 | 1.25 | 0.0 | N/C |
S. pyrogene | 5.0 | 5.0 | 10 | 10 |
P. aeruginosa | 10 | TNC | TNC | TNC |
C. albicans | TNC | TNC | TNC | TNC |
TNC - Test not conducted | ||||
3.3. Isolation and characterization
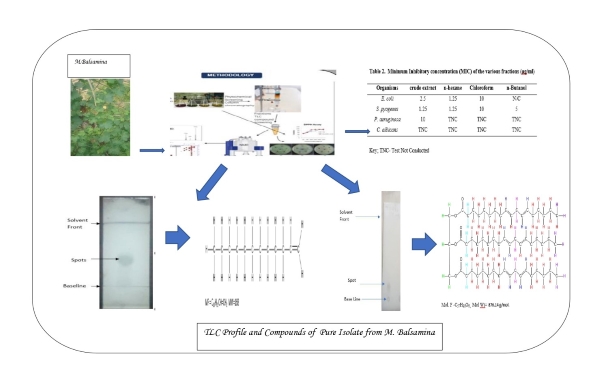
A total of 37 pooled fractions (10 mL each) were collected. Notably, fraction 24 (5g) was further split, yielding 10 smaller fractions (24-A to 24-J). Thin layer chromatography of fraction 24-A revealed two distinct spots, indicating two compounds. The yellow spot identified via a general spray reagent with 5% sulphuric acid was purified using preparative thin layer chromatography (PTLC) to isolate compound ED-A (100 mg), while fraction 24-B yielded ED-B (40 mg). The respective Rf values were 0.65 and 0.3. This is shown in Fig. 1 [20, 30-32].
Figure 1. Thin layer chromatography (TLC) plate showing a single of ED-A (n-hexane-ethylacetate (95:5).
Compound ED-A is a light yellow oily liquid (75.2 mg), identified as dodec-1-ene. GC/MS analysis revealed fragmentation patterns typical of α-olefins, with a mass spectrum showing peaks at m/z 167 and fragments at m/z values of 167, 140, 126, 111, 97, 91, 83, 69, and 55 (Fig. 2.) The 1H NMR spectrum (850 MHz) displayed signals at δH 0.91 ppm for terminal methyl protons and multiplet peaks at δH 1.30-1.35 ppm for methylene protons. Vinylic proton signals appeared at δH 5.01 and 4.99 ppm, while allylic protons were observed at δH 2.08-2.13 ppm (Table 5). The 13C NMR showed twelve carbon resonances (Table 5), confirming the presence of sp2 and sp3 hybridized carbons. The molecular formula of dodec-1-ene is C10H21CH=CH2, with a molecular mass of 168 g/mol [32, 33].
Figure 2. GC-MS Spectrum of ED-A.
Table 5. Chemical shift values for (C6D6) spectra data of ED-A
Assignment | 13C NMR (ppm) | Carbon Types | 1H NMR (ppm) | ||
ED-A | Ref-1 | ED-A | Ref-1 | ||
1 | 114.05 | 114.46 | CH2 | 4.99, d, 5.01, d | 4.96 d |
2 | 138.90 | 130.05 | CH | 5.23-5.35, m | 5.02, m |
3 | 33.91 | 34.22 | CH2 | 2.08-2.13, m | 2.05, m |
4 | 22.80 | 23.08 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
5 | 29.03 | 29.36 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
6 | 29.24 | 29.56 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
7 | 29.52 | 29.75 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
8 | 29.64 | 29.91 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
9 | 29.82 | 30.09 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
10 | 29.89 | 30.09 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
11 | 32.02 | 32.32 | CH2 | 1.30-1.35, m | 1.26-1.47, m |
12 | 14.05 | 14.50 | CH3 | 0.91. t | 0.88, t |
d=doublet, t=triplet, m=multiplet, ppm= parts per million, Ref-1= [30, 31] | |||||
Compound ED-B (Table 6) was isolated as a white amorphous powder (45 mg) with an Rf value of 0.3. Its 1H NMR spectrum (850 MHz, CDCl3) exhibited signals characteristic of triglycerides (18:2, 18:2:18:2), indicating the presence of unsaturated fatty acids. Notably, vinylic protons were observed at δH 5.12 ppm, while glycerol protons appeared at δH 4.01-4.13 ppm. Other relevant signals included bis-allylic protons at δH 2.27-2.29 ppm and terminal methyl groups at δH 0.87-0.88 ppm. The 13C NMR (213 MHz, CDCl3) revealed four resonance clusters, including carbonyl peaks (δC 173.75-174.09 ppm) and olefinic regions (δC 124.32 ppm). DEPT-135 NMR confirmed the presence of three quaternary carbons and various methylene and methyl carbons. Homonuclear and heteronuclear correlation spectroscopy further established relationships between protons and carbons. Ultimately, ED-B was identified as trilinolein (C57H98O6), a triglyceride formed from glycerol and three linoleic acid chains [30, 34, 35].
Table 6. Chemical shift values for (CDCl3) spectra data of ED-B
Assignment | 13C NMR (ppm) |
| 1H NMR (ppm) | Types of carbon/proton | ||
ED-B | Ref-1 | ED-B | Ref-1 |
| ||
1 | 174.99 | 173.32 |
| - | - | C=O |
1’ | 173.09 | 172.87 | - | - | C=O | |
1” | 173.75 | 173.32 | - | - | C=O | |
2 | 34.43 | 34.06 | 2.41-2.43 | 2.33-2.25 | CH2 | |
3 | 25.96 | 24.88 | 1.60-1.67 | 1.62 | CH2 | |
4 | 29.19-29.73 | 29.10 | 1.25-1.29 | 1.27-1.31 | CH2 | |
5 | 29.19-29.73 | 29.29 | 1.25-1.29 | 1.27-1.31 | CH2 | |
6 | 29.19-29.73 | 29.14 | 1.25-1.29 | 1.27-1.31 | CH2 | |
7 | 29.19-29.73 | 29.65-29.68 | 1.25-1.29 | 1.27-1.31 | CH2 | |
8 | 28.66 | 27.23 | 2.00-2.05 | 2.03-2.04 | CH2 | |
9 | 124.32 | 129.69-130.03 | 5.12 | 5.36 | -CH=CH- | |
10 | 124.32 | 128.08 | 5.12 | 5.36 | -CH=CH- | |
11 | 29.19-29.73 | 29.71 | 5.12 | 5.36 | CH2 | |
12 | 124.32 | 127.90 | 5.12 | 5.36 | -CH=CH- | |
13 | 124.32 | 130.24 | 5.12 | 5.36 | -CH=CH- | |
14 | 28.66 | 27.19 | 2.00-2.05 | 2.03-2.04 | CH2 | |
15 | 29.19-29.73 | 29.38 | 1.25-1.29 | 1.27-1.31 | CH2 | |
16 | 32.96 | 31.94 | 1.25-1.29 | 1.27-1.31 | CH2 | |
17 | 22.73 | 22.70 | 1.25-1.29 | 1.27-1.31 | CH2 | |
18 | 14.28 | 14.13 | 0.87-0.88 | 0.88-0.90 | CH3 | |
Glycerol (CH2O-COR) | 64.43 | 62.10 | 4.01-4.13 | 4.14-4.18 | CH2 | |
Glycerol (CHO-COR) | 61.18 | 68.88 | 4.01-4.13 | 5.28 | CH2 | |
Ref-1= [20] | ||||||
4. Conclusions
In conclusion, this study has demonstrated that Momordica balsamina leaves contain both primary and secondary phytochemicals with dose-dependent antimicrobial activity. This finding underscores the potential of this plant in traditional medicine and the pharmaceutical industry. Isolation and structural identification of compounds ED-A and ED-B were successfully conducted from the n-hexane fraction of the leaves using chromatographic techniques. Their structures were elucidated through a combination of physical and spectrophotometric analyses. Specifically, ED-A was confirmed to be dodec-1-ene, while ED-B was identified as a trilinolein triglyceride.
Authors’ contributions
Conceptualization, E.J., H.B.Y.; Literature search and data extraction, E.J., H.B.Y., A.B.B.; Experimental analysis and interpretation of the findings, E.J., H.B.Y., A.B.B.; Drafted the initial manuscript, E.J., H.B.Y.; Substantial revisions and edits, E.J., H.B.Y., A.B.B., G.M.T.; Formatting and proofreading, E.J., H.B.Y., A.B.B.; Final manuscript revisions and ensured consistency across sections, E.J., H.B.Y., A.B.B.
Acknowledgements
A special thanks goes to the Department of Pharmaceutical Chemistry, University of Maiduguri, Nigeria, for supplying the laboratory with the required materials used for isolation. Also, we thank the NMR laboratory of the Saudi Arabia University of Science and Technology for availing their facility for the NMR studies.
Funding
This research received no external funding.
Availability of data and materials
All relevant data are within the paper and its supporting information files. Additional data will be made available on request according to the journal policy.
Conflicts of interest
The authors declare no conflict of Interest.
References
|
1. |
Dirar, A.I.; Devkota, H.P. Ethnopharmacological uses,
phytochemistry and pharmacological activities of Guiera senegalensis J.F.
Gmel. (Combretaceae). J.
Ethnopharmacol. 2020, 113433.
https://doi.org/10.1016/j.jep.2020.113433 |
|
2. |
Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G.
Antifouling and antimicrobial biomaterials: an overview. J. Pathol.
Microbiol. Immunol. 2017, 125(4), 392–417.
https://doi.org/10.1111/apm.12675 |
|
3. |
World Health Organization (WHO). Herbal medicine research
and global health: An ethical analysis. WHO, 2011. |
|
4. |
Sarwar, M.; Attitall, I.H.; Abdollahi, M. A review on the
recent advances in pharmacological studies on medicinal plants, animal
studies are done but clinical studies needs completing. Asian J. Anim. Vet. Adv. 2011,
6(8), 867-883.
https://doi.org/10.3923/ajava.2011.867.883 |
|
5. |
Iwu, M.M. Handbook of African Medicinal Plants. CRC Press,
Taylor and Francis Group, London, 2, 2, 2014. |
|
6. |
Uhegbu, F.O.; Imo, C.; Ugbogu, A.E. Effect of aqueous
extract of Piper guineense seeds on
some liver enzymes, antioxidant enzymes and some hematological parameters in
Albino rats. Int. J. Plant Sci. Ecol. 2015, 1(4),167–171. |
|
7. |
Parveen, A.;
Parveen, B.; Parveen, R.; Ahmad, S. Challenges and guidelines for clinical
trial of herbal drugs. J. Pharm. Bioal. Sci. 2015, 7(4), 329-333.
https://doi.org/10.4103/0975-7406.168035 |
|
8. |
Ota, A.;
Ulrih, N.P. An overview of herbal products and secondary metabolites used for
management of type two diabetes. Front. Pharmacol. 2017, 8, 436. https://doi.org/10.3389/fphar.2017.00436 |
|
9. |
Ekeopara,
C.A.; Azubuike M.I. The contributions of African traditional medicine to
Nigeria’s health care delivery system. IOSR J. Human. Soc. Sci. 2017, 22(05),
32–43. https://doi.org/10.9790/0837-2205043243 |
|
10. |
Raphael, E.C. Traditional medicine in Nigeria: Current
status and the future. Res. J.
Pharmacol. 2011, 5(6), 90-94.
https://doi.org/10.3923/rjpharm.2011.90.94 |
|
11. |
Kunle, O.F. Standardization of herbal medicines -A review.
Int. J. Biodivers. Conserv. 2012, 4(3), 101–112.
https://doi.org/10.5897/ijbc11.163 |
|
12. |
Bello, A.; Muhammad, F.; Kankara, S.S.; Abdulkadir, B.;
Buhari, Y. Antimicrobial activity of Balsam Apple (Mormodica balsamina L.). J. Microbiol. Res. 2018, 3(1), 24–29. https://doi.org/10.47430/ujmr.1831.004 |
|
13. |
Ezzat, S.M.; Jeevanandam, J.; Egbuna, C.; Kumar, S.;
Ifemeje, J.C. Phytochemicals as sources of drugs. Phytochemistry: An
In-silico and In-vitro Update. In Springer eBooks, 2019, 3-22. https://doi.org/10.1007/978-981-13-6920-9 |
|
14. |
Nursuhaili, A.B.; Nur, A.; Syahirah, P.; Martini, M.Y.;
Azizah, M.; Mahmud, T.M.M. A review: Medicinal values, agronomic practices
and postharvest handlings of Vernonia
amygdalina. Food Res. 2019, 3(5), 380–390.
https://doi.org/10.26656/fr.2017.3(5).306 |
|
15. |
Tcheghebe, O.T.; Signe, M.; Seukep, A.J.; Ngouafong, F.;
Abstract, T. Review on traditional uses, phytochemical and pharmacological
profiles of Garcinia kola Heckel.
Merit Res. J. Med. Med. Sci. 2016, 4(11), 480–489. |
|
16. |
Evans, W.C.
Phytochemicals in Trease and Evans’ Pharmacognosy, 16th Edition. Saunders Elsevier Toronto, Canada. 2009,
pp.1-9, 26, 225, 252, 304, 356, 437-440. |
|
17. |
Sofowora, A. Screening plants for bioactive agents. In:
Medicinal plants and traditional medicine in Africa. 2nd Edition,
Spectrum Books Ltd., Sunshine House, 1993, 134-156. |
|
18. |
Rubina, L.; Firdaus, J.; Vinod, K.; Mohd, J. Evaluation of
antibacterial activity of plant extracts on antibiotic susceptible and
resistant Staphylococcus aureus
strains. J. Chem. Pharm. Res. 2011, 3(4), 777-789. |
|
19. |
Buchanan, R.L.; Phillips, C. NMR
spectroscopy for the characterization of lipids. J.
Lipid Res. 2003, 44(8), 1507-1520. |
|
20. |
Wu, X.; Xu, L.;
Yuan, G.; Wang, Y.; Xu, X. Triglycerides isolated from Streptomyces
sp. ZZ035 and their nuclear magnetic resonance spectroscopic characters. Spect. Anal. Rev. 2017, 5, 1-10. https://doi.org/10.4236/sar.2017.51001 |
|
21. |
Jaichand, V.; Mellem, J.J.; Mohanlall, V. The proximate composition and phytochemical
screening of Momordica balsamina
(balsam apple) fruit and leaves. Food Sci. Technol. 2024, 44, 177. https://doi.org/10.5327/fst.00177 |
|
22. |
Osuntokun, O.T.; Ajayi, A.O. Antimicrobial, phytochemical
and proximate analysis of four Nigerian medicinal plants on some clinical
microorganisms. Curr. Res.
Microbiol. Biotechnol. 2014, 2(5),
457-461. https://doi.org/10.1038/nbt1082 |
|
23. |
Thakur, G.S.; Bag, M.; Sanodiya, B.S.; Bhadouriya, P.;
Debnath, M.; Prasad, G.B.; Bisen, P.S. Momordica
balsamina: A medicinal and neutraceutical plant for health care
management. Curr. Pharm. Biotechnol. 2009, 10(7), 667–682.
https://doi.org/10.2174/138920109789542066 |
|
24. |
Ramalhete, C.; Gonçalves, B.M.F.; Barbosa, F., Duarte, N.;
Ferreira, M.U. Momordica balsamina:
Phytochemistry and pharmacological potential of a gifted species. Phytochem.
Rev.: proceedings of the Phytochemical Society of Europe. 2022, 1(2),
617–646. https://doi.org/10.1007/s11101-022-09802-7 |
|
25. |
Nogbou, N.; Mabela, D.R.; Matseke, B.; Mapfumari, N.S.;
Mothibe, M.E.; Obi, L.C.; Musyoki, A.M. Antibacterial activities of Monsonia Angustifolia and Momordica balsamina Linn extracts
against carbapenem-resistant Acinetobacter
Baumannii. Plants. 2022, 11(18), 2374.
https://doi.org/10.3390/plants11182374 |
|
26. |
Kooshari, H.; Ghaemi, F.A.; Sadegh, M.S.; Jahedi, M.;
Zahiri, M. The investigation of antibacterial activity of selected native
plants from North of Iran. J. Med. Life. 2015, 8(2),38-42. |
|
27. |
Abdulhamid, A.; Sanusi A.J.; Sani, I.; Bagudo, A.I.;
Abubakar, R. Phytochemical and antibacterial activity of Mormodica balsamina leaves crude extract and fractions. Drug
Discov. 2023, 17(39), 1–8. https://doi.org/10.54905/disssi.v17i39.e11dd1012 |
|
28. |
Andrews, J.M. Determination of minimum inhibitory
concentrations. J. Antimicrob.
Chemother. 2001, 48(1), 5–16. https://doi.org/10.1093/jac/48.suppl_1.5 |
|
29. |
Otimenyi, S.O.; Uguru, M.O.; Ogbonna, A. Antimicrobial and
hypoglycemic effects of Momordica
balsamina. Linn. J. Nat. Prod. 2008, 1, 03-09. http://hdl.handle.net/123456789/3130 |
|
30. |
Gunstone, F.D.
Fatty
acid and lipid chemistry. Edinburgh: The Royal Society of
Chemistry. 2004. |
|
31. |
Ghosh, S.; Choudhury, M.D. Applications of 1H NMR spectroscopy in
the study of lipids. Adv. Res. Life Sci. 2018,
5(1), 15-25. |
|
32. |
Fuchs, B.; Metzger, J.W. Spectroscopic
techniques for the characterization of lipids. Lipids.
2003, 38(5), 527-536. |
|
33. |
Schmidt, R.R.;
Koller, W. Identity and analysis of
α-olefins by GC/MS. J. Chromatogr. A.
2000, 885(1-2), 113-124. |
|
34. |
Wang, R.; Chen, J.
Nuclear magnetic resonance (NMR) spectroscopy: Applications in Organic
Chemistry. Comprehens. Org. Chem.
II,2012, 1, 425–458. |
|
35. |
Pavia, D.L.; Lampman,
G.M.; Kriz, G.S. "Introduction to
Spectroscopy." Houghton Mifflin
Company, 2015. |
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
This
study took a closer look at the leaves of Momordica balsamina, a plant
long valued in African folk medicine. The leaves were extracted using methanol
and a gentle cold maceration method. Then, it was analyzed for its chemical
composition and carefully separated into different fractions: n-hexane,
chloroform, and n-butanol. The extracts were tested against common pathogens
like E. coli,
S. pyogenes,
P. aeruginosa,
and C. albicans.
The results were found to be promising, with better inhibition of E. coli
and S. pyogenes
at 12.5 μg/mL present in the n-hexane fraction.
The MBC result shows the crude extract and the n-hexane fraction being
bactericidal against E. coli and S. pyogenes at 1.25
μg/mL and 5 μg/mL, the
phytochemical analysis revealed a variety of beneficial compounds, including
carbohydrates, steroids, and flavonoids, while noting that anthraquinone was
absent. The extract and its n-hexane fraction showed effectiveness against
E. coli
and S.
pyogenes, but had only a minimal impact on P. aeruginosa
and no significant antifungal effects. Interestingly, the
research led to the isolation of two compounds—dodec-1-ane (C10H21
CH=CH2) and trilinolein glyceride (C57H98O6)—for
the first time from M.
balsamina leaves.
Overall, these findings reinforce the plant's traditional use in treating
infections and suggest it could be a valuable source for developing new
antimicrobial treatments.
Abstract Keywords
M. balsamina leaves, cold maceration, pathogens, phytochemicals, dodec-1-ene, trilinolein glyceride.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


