Review Article
George Dzorgbenya Ametefe
George Dzorgbenya Ametefe
Corresponding
Author
Department of Chemical Sciences, Biochemistry Unit, Oduduwa University, Ipetumodu, Osun State, Nigeria.
E mail: ametefegeorgee1@gmail.com, Tel: +234-8034833599
Frank Anayo Orji
Frank Anayo Orji
Abstract
Pectin is known as a polymer of galaturonic acid.
Pectins are commonly found in plants due to the presence of cell walls; with
the pectins categorised into different classes, owing to the differences of
their solubility in water, percent methyl group and carboxyl group
esterification of the galacturonate units. Pectinases (pectin enzymes) are
biocatalysts that split pectin into simpler forms. Over the years, the
production of this enzyme has been discovered from inexpensive agro wastes and
environmental microorganisms which have been evaluated for their specific
capacities to effectively generate this enzyme. The production of pectinase via fermentation is made possible using
the substrate, pectin, an inducer. Fermentation is the primary mode for pectinase production; as
microorganisms employed in the fermentation process act by degrading the more
complex substrate into simpler forms with the production of energy. Solid-state
and submerged fermentations are the main fermentation methods employed in the
industrial production of pectinases. The existence of the different
types of pectin is indicative of the corresponding pectinases produced, and the
justification for the mode of action during catalysis. Several factors have
been implicated in pectinase production, such as the type of fermentation
method used (solid-state fermentation or submerged fermentation), others are;
the substrate type employed in fermentation, pH, temperature, duration of
fermentation, substrate composition, type of metal ion, extraction solvent
used, and the type of precipitation method used for concentrating the enzyme.
This review gives an overview of pectin and processes leading to the production
of the enzyme and simplifies some major modes of action involved in the
application of these enzymes and some relevant factors for optimum production
and application of the enzyme. The
review also shows advances in the extraction of pectin. Some challenges and next steps for future research
have also been pointed out.
Abstract Keywords
Pectin, pectinase, agro-wastes, fermentation, microorganisms.1. Introduction
Pectin is a heteropolysaccharide present in plant cell walls; it is mostly made up of galacturonic acid, which is a naturally occurring sugar generated from galactose [1]. This naturally occurring sugar is present in most resources such as agricultural wastes, which were formerly underutilized. In most areas, 'agro wastes' have opened up room for the production of enzymes from some components of the plant cell wall such as pectin, cellulose, and lignin, among others [2, 3]. These plant cell wall components have enabled them for use in vast areas as feedstock and source of energy as they are abundant and cheap. Encouragingly, a host of the vastly available substrates (from agro wastes) are utilized by microorganisms as energy sources and for the production of enzymes [4]. Waste in this context is therefore defined as reusable materials assuming characteristics of co-products or by-products.
When the degree of esterification is
greater than 50%, pectin extract is categorized as high methoxyl or high ester
pectin; however, if the degree of esterification is less than 50 %, the pectin
is classed as low ester or low methoxyl pectin [5, 6]. The degree of
esterification is defined as the proportion of D-galacturonic acid carboxyl
groups that have passed through the esterification process with ethyl alcohol [123]. Amidated pectin is produced by reacting
ammonia with carboxymethyl groups (-COOCH3) on the pectin molecule [7, 8]. The
percentage of pectin carboxylic acid groups present in amide form is
characterized as the degree of amidation (DA). Some properties of pectin gels
are altered when methoxyl groups are replaced with amide groups; for example,
amidation enhances pectin water solubility [9] and
allows it to be more thermoreversible and sustain more calcium variability [10-12].
The 2023 world population was estimated
to be 8.01 billion, rich in natural resources accompanied by an increase in
abundance of resultant waste generation across the many continents of the world
[13].
Markets, streets, and some residential
facilities are also littered with plant wastes such as the cobs from corn,
fruit peels, and vegetable wastes, among others. The inadequate sorting of
these agricultural wastes from other forms of waste and their employment in the
transformation of wastes (raw materials) to wealth (finished goods) is a
challenge. Pectin, a substrate for pectin enzyme (pectinase) production can be derived
from these plant wastes [14]. The
pectin enzyme (pectinase) can be produced from these natural resources for
industrial production such as the clarification of fruit juices [14-16]. The study harvests relevant literature
that addressed the classification of pectin (the substrate for pectinase
production); the mode of action of the enzyme and factors influencing pectinase
production.
2. Materials and methods
Related articles were the electronic materials sourced for the review from 2014 to 2023 (the last decade). The method of search showed that publications considered were review & research articles in the fields of Agricultural and Biological Sciences &Biochemistry, Genetics and Molecular Biology and were Open access & Open archive (Chart 1). The method used for the elimination of articles was undertaken based on duplication of the same or closely similar findings and the relevance of the articles to the study from mostly the myriad of articles on ScienceDirect database as shown in the charts in Figs. 1 & 2, and Charts 1 & 2. The filters used are as indicated in Figs. 1-2.
Figure 1. Systematic review flow chart for
protopectin on ScienceDirect database
Figure 2. Systematic review flow chart for
pectinase on ScienceDirect database
Chart 1. Pictorial representation of search for Figure 1.
Chart 2: Pictorial depiction of search for Figure 2.
3. Results and discussion
3.1 Pectin and its extraction
Pectin is a complex heteropolysaccharide and can be found in the cell walls and middle lamella of plants. This complex heteropolysaccharide is used as a stabilizer, encapsulant, hydrocolloid, and gelling agent in foods [17]. Pectin can be extracted from fruits using the ‘precipitation and alcohol’ method by Maskey and colleagues [18]. In the Maskey and colleagues study, distilled water was measured in a conical flask, the temperature was raised to 120 oC with the addition of plant pulp powder, and the pH lowered with acid to 1.5; the mixture was stirred and extracted for about 60 minutes. The mixture was then allowed to cool to 55 oC in an ice water bath before centrifuging for 10 minutes at 257g and vacuum filtering with Whatman filter paper. Alcohol was added to the filtered solution, and precipitation was allowed overnight. The next day, pectin was separated from the alcohol solution using Muslin cloth before being dried in an oven [18]. For instance, another study showed that bell pepper and carrot powder were washed twice in 2.5 hours with 85% aqueous ethanol at ambient temperature and once in 1.5 hours at 80 oC for lab-scale extraction of polysaccharide-rich fractions. Centrifugation was used to separate the alcohol-insoluble residue (AIR). The polysaccharides (PS) were extracted by boiling the AIR twice in water for 3 hours (air: water ratio approx. 10), then combining and lyophilizing the aqueous phases. This substance is known as bell pepper and carrot extract. Polysaccharide extract was diluted in 35 mM sodium acetate pH 5.0, and MACER 8W, a polysaccharide degrading enzyme mixture with broad specificity (Biocatalysts Ltd., Cardiff, UK), was added. The reactions were allowed to run for 24 hours at 37 degrees Celsius before being halted by boiling for 5 minutes. 1H-NMR spectroscopy confirmed polysaccharide hydrolysis; followed by saponification of the polysaccharide extracts and solid-phase C-18 silica of the polysaccharide samples [19]. Other advances in pectin extraction using innovative approaches can be found in the review of Kumar et al. [17], as the authors stated that, microwave, ultrasonic, and enzyme-assisted extraction technologies are ecologically friendly and efficient. Extraction of pectin using ‘superfine grinding pretreatment’ and the use of ‘surfactant and microwave-assisted processes have also been investigated with a good yield of pectin [3, 20]. A review of pectin content in various fruits has been reported such as apple, banana, carrot, guava, lemon pulp, mango, pineapple, and strawberries among others [21]. The antioxidant and anti-inflammatory effects of carob pectin extract after drying and grinding have been demonstrated in conjunction with some polyphenols. [1].
A similar process for extracting pectin is utilized using alcohol. It is the preferred method for pectin extraction; however, both the conventional heating method with an oven and electromagnetic induction (EMI) heating have been used. The yield of pectin was calculated based on the ratio of the dried weight of pectin to the weight of the dried fruit. The result showed that electromagnetic induction (EMI) heating resulted in a higher yield of pectin than the conventional oven heating method [22]. Prior to the EMI heating method, pretreatment of the raw material is critical for removing low molecular carbohydrates, color pigments, and organic chemicals, and inactivating pectic enzymes, as it involves washing and drying of the albedos followed by the dried residues treated with ethanol and filtering and drying to give the alcohol insoluble solids (AIS). A part of the AIS was suspended in acidic pH water for approximately 90 minutes in magnetizable and enameled containers put on an induction plate, with constant stirring [22].
Figure 3. Structure of pectin (Kdo, 3-DeoxyD-manno-2-octulosonic acid;
DHA, 3-deoxy-D-lyxo-2-heptulosaric acid. HG and RGI are much more
abundant than the other components) [23].
The four distinct polysaccharide types that makeup pectin are displayed along with their structures (Fig. 3). Kdo stands for 3-DeoxyD-manno-2-octulosonic acid, and DHA for 3-Deoxy-D-lyxo-2-heptulosaric acid. Compared to other components, HG and RGI are significantly more prevalent.
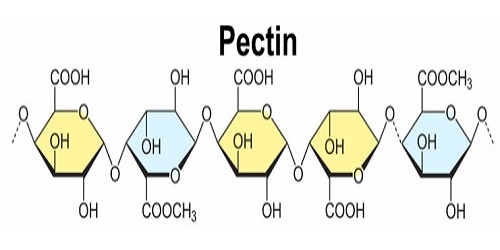
The application of pectin in drug delivery has also been uncovered along with the nanoemulsion formation. This application is made possible because of the structure (Fig. 4) having several hydroxyl and carboxyl groups along its backbone thereby enabling its functionalization and ease of modification with other bioactive compounds [17].
Figure 4. Structure of pectin (Source: https://healthjade.net/pectin)
3.2 Classification of pectin
Of the many classification types, the American Chemical Society classifies pectic substances as:
1. Protopectin: this constitutes the substances of pectin origin that are insoluble in water, and the products obtained from protopectin hydrolysis are pectin or pectic acids [24-28].
2. Pectic acid: this is part of the polymer of galacturonans, which are soluble but contain very little or no noticeable number of groups of methoxy origin. So, acids or normal salts of pectic acid are known as pectates [24].
3. Pectinic acid: this makes up more than 'zero and less than 75% methylated galacturonate units. Pectinates are known to be normal or acid salts of pectinic acids [24].
4. Pectin (polymethyl galacturonate): not less than 75% of the carboxyl groups of the galacturonate units are esterified with methanol on the polymeric material. This explains the rigidity of the cell wall when bound to cellulose, thereby showing the covalent linkage of pectin to cellulose [29,30].
In nature, pectin occurs in the insoluble form in fruits that are not ripe and bound to cellulose microfibrils, therefore accounting for the cell wall rigidity [2]. The alteration of pectin structure in fruits by pectin enzymes leads to the breaking of the pectin bonds. The formation of pectin gel structure results from the 3-dimensional crystalline network in pectin obtained from the cross-linking of the portions of D-galacturonic acid [31].
Muller-Maatsch et al. [32] studied the amount of pectin in twenty-six food wastes; orange peels, onion hulls, fresh pumpkins, apple pomace, a whole apple, and tomato skins, among other wastes, with the report of different contents of calcium-bound pectins to ester rich pectins. The presence of multiple hydroxyl and carboxyl groups throughout the backbone of pectin gives it a distinctive structure that allows for easy modification and functionalization with diverse bioactive substances [17]. The need for enzymes to split the bonds in pectins using enzymes.
The existence of living things is aided in no small measure by the chemical reactions occurring in nature. Though some of the naturally occurring reactions could take place without enzymes, however, enzymes act as catalysts to enhance such reactions hence affecting in no small measure the sustenance of life [33,34]. Thus, the simple definition of enzymes as biocatalysts that increase the rate of reactions, indicates that not only humans are endowed with these vital substances; microorganisms also secrete enzymes while feeding.
It is evident in the symbiotic relationships humans share with microorganisms in the gut, where the microorganisms understandably secrete enzymes to further degrade the undegraded materials in the colon before their expulsion from the body [35, 36].
It is expedient to say that enzyme action has been exploited for many years, such as in the production of alcohol, where fermentation occurred by imploring microorganisms like fungi; however, until reasonably recent in enzyme application, the roles of enzymes have been understood [37, 38]. The understanding of the functions of an enzyme in biological reaction processes has now aided the merging of this knowledge with other fields of human endeavors such as engineering, and computer science programming for the design and construction of instruments to further optimize its production for human needs [39]. Many enzymes exist as numerous studies have engaged this field, and more insights are still revealed for industrial applications, among other needs. The clamor for enzymes is mainly due to the ability of enzymes to be specific in their mode of action, their high precision, the need for a relatively mild environment for their action, and not forgetting their environmentally friendly characteristic (known fact).
The reason microorganisms are predominantly used is that microorganisms produce enzymes in shorter duration as compared to other sources for industrial production [40]. Various microorganisms have been utilized to produce enzymes in the industries comprising eukaryotic and prokaryotic microorganisms, such as fungi, yeasts, and bacteria in enzyme production for multiple purposes [41].
3.4 Pectinases (pectin enzymes)
Pectinases could be described as an enzyme group that through hydrolysis, trans-elimination, and de-esterification reactions, act on pectin and hydrolyze the ester bond between methyl and carboxyl pectin groups [42]. So, pectinases are enzymes that degrade pectin [43, 44]. They are embedded with high catalytic properties [45]. Microbial pectinases account for about 25% of the global food enzyme sales and are useful for clarifying fruit juices [7]. Numerous microorganisms like yeasts, bacteria, and molds produce pectinase [43].
3.4.1 Classification of pectinases
a). Protopectinases
This class of pectinases acts on the protopectin substrate and makes it soluble by polymerization [29].
b). Pectin Methyl Esterases (PME)
PME, through de-esterification of the methoxy pectin group, produces methanol and pectic acid; so, it selectively first acts on a methyl ester galacturonate unit group close to a non-esterified galacturonate unit. So, PME exhibits its action before polygalacturonases and pectate lyases, as these enzymes act on substrates that are not esterified [50, 51].
c). Polymethylgalacturonases (PMG)
They are known to catalyze the splitting of α-1, 4-glycosidic bonds (Fig. 5), with a preference for the more esterified pectin giving rise to 6-methyl-D-galacturonate [31]. Saccharomyces cerevisiae is known for this pectinase production [14]. Both the exo and endo polygalacturonase are the two main pectin enzymes for the complete degradation of the substrate to a simple form (i.e., the monomer of pectin) [46].
d). Polygalacturonases (PG).
PG catalyzes the hydrolysis of α-1, 4-glycosidic linkages in the polymer of galacturonic acid (i.e., polygalacturonic acid), thereby producing D-galacturonate. PG and PMG act in both the endo and exo modes. Simultaneously, random substrate cleavage is catalyzed by endo-PMG and endo-PG, exo-PG, and exo-PMG act by hydrolytically catalyzing the splitting of the non-reducing end of the substrate [46].
e). Pectate lyases (PGL)
PGL acts by catalyzing the cleavage of the glycosidic linkages with a preference for polygalacturonic acid-producing unsaturated products [2].
f). Pectin Lyases (PL)
Pectin lyases catalyze the random cleavage of pectin by acting on the more esterified pectin (Fig. 5), which via trans-elimination of glycosidic linkages produce unsaturated methyl-oligogalacturonates [31].
3.4.2 Modes of action of pectinases
The existence of different forms of pectin accounts for the differences in the forms of pectinase existence and their unique modes of action.The reason for the existence of the various types of pectinases further shows that polygalacturonase (PG); pectinesterase (PE); polymethyl galacturonase (PMG), and pectin lyase (PL) exist due to their ability to act on their unique substrates. Pectinases can be classified into three types: they include; protopectinases, de-esterifying enzymes (pectin esterases), and depolymerizing enzymes (pectinases, hydrolases, and lyases) [29]. As depolymerase split β- (1, 4) glycosidic bonds between monomers of galacturonic acid in the pectin substrates via hydrolysis or β-elimination (lyases), pectin esterases, and protopectinases as described above, lyases through trans-elimination cleave the polymer of galacturonic acid [47].
Figure 5. Modes of action of pectinases [35]
a) R=H for PG and CH3 for PMG; b) PE cleaves the bond between COO- and CH3, and c) R=H for PGL and CH3 for PL (Fig. 5).
NOTE: The arrow shows the site or sites for the various pectinase actions with the substrates. PG, polygalacturonase; PE, pectin esterase; PMG, Polymethylgalacturonase and PL, pectin lyase [48].
3.4.2.1 Protopectinases
These are a diverse class of enzymes known to liberate pectins soluble in water from the protopectin in the tissue of plants [49]. In other words, when protopectin in the presence of water reacts with protopectinase, the substrate pectin, which is soluble in water, is released.
A study to examine the expression of gene changes in the growth of banana from the unripe stage all through the ripening process obtained that the unripe banana had more of the proto pectin than the ripe banana; thereby giving credence to the function of proto pectinase action in the unripe stage [50].
3.4.2.2 De-esterifying pectin enzyme (pectin esterases)
Pectin esterases or pectin methylesterases are referred to as pectin methyl esterases [31]. This enzyme is utilized in the catalysis of the removal of ester in methyl ester linkages in the backbone of the pectin, thereby releasing methanol and pectic substances that are acidic [51]. The product from the de-esterification process is then acted upon by lyases [52, 53].
3.4.2.3 Depolymerizing enzymes (pectinases; hydrolases, and lyases)
A. Hydrolases
These are mainly for the hydrolysis of the soluble pectin (that is, in the presence of water). Depolymerases can be produced by microorganisms such as bacteria, fungi, and yeasts. Additionally, enzymes such as some cellulases and pectin enzymes catalyze reactions by hydrolysis. Reducing sugars such as glucose are released and measured to determine the activity of such enzymes on their respective substrates [31]. A variety of hydrolases exists, some of which are:
a. Polygalacturonases (PG)
These are enzymes that split the polymer of galacturonic acid by introducing water. They are the predominantly studied subtype of hydrolases in comparison to other pectin enzymes. The subtype of PGs known to involve reactions could split polygalacturonic acids via the exo- or the endo-modes [54]. While the endo polygalacturonases are mostly secreted by microorganisms such as bacteria, fungi, and some species of yeasts; they are found in some plants and nematodes that are parasitic [55-58].
b. Oligo galacturonate hydrolase
This type of hydrolase enzyme is known to hydrolyze the substrate oligo galacturonates; this enzyme, in comparison to endo polygalacturonases, is secreted via autolysis [59].
c. Lyases
Lyases are known to give rise to unsaturated products.
3.5. Sources of pectinases
The drive towards operations that reduce global warming is giving rise to embracing green biotechnology; hence, enzymes from microbial origin have led to the realization of this drive by excluding harmful alternatives [60]. Microorganisms are the primary organisms utilized for industrial-scale production of pectinase, with yeasts and molds contributing to about 50%, bacteria 35%, and plants and animal pectinases accounting for 15% [61]. Aspergillus niger from the literature is reported as being the most studied microorganism for pectinase production. However, yeasts such as Saccharomyces cerevisiae have also been used to produce pectinase and applied in the processing of fruit juice [14].
Alkaline and acidic pectinases are secreted by prokaryotic and eukaryotic microorganisms, respectively [31]. Though bacteria also produce pectin enzymes, many of these bacteria are not in the class of microorganisms regarded as safe; that is, many of these bacteria do not belong to the class Generally Regarded as Safe with the acronym GRAS [62]. It is also essential to recognize the production of enzymes without the need for fermentation as the endogenous extraction of pectinase from organisms has been reported [63, 64].
3.6. Isolation and screening of pectinolytic microorganisms
It is now common knowledge that pectinolytic microorganisms are sourced from our immediate environment [65]. A common method of screening for pectinolytic microorganisms based on the zone of clearance has been the preferred method of choice. A variety of modifications to the method have been employed; of which, recent studies were based on the microbial ability of each isolate to form a zone of hydrolysis or clearance before its molecular identification [66-70]. Before the microbial screening for confirming pectinase secretion potential, the microorganisms are usually isolated from the environment using also simple microbiology techniques, where an environment containing the deteriorated substrates (pectins) is carefully collected and transported to the laboratory for isolation of the variety of microorganisms present. After that, the samples were ground for ease in obtaining the microbial population in each deteriorated sample. Dilution is usually undertaken to extract the microorganisms in the solvent and further dilutions are to reduce the microbial community, thereby reducing the challenge of microbial identification of cultured colonies. Potato Dextrose Agar (PDA) is generally used as a suitable medium for the isolation process of the microorganisms using the pour plate method and incubated at room temperature for usually about two to five days [71].
After that, each colony formed a sub-cultured for the derivation of pure culture for each microbial isolate. The sub-cultured microbial populations are screened on media preparation containing pectin as the only source of carbon and acting as the inducer; such as in the modification of Czapek medium by substituting the carbon source (cellulose) as used in Chinedu et al. [72] with pectin and incubation of each of the pure isolated microbe left for a few days [73].
After that, the formation of ‘spherical’ zones often referred to as zones of hydrolysis or clearance, is observed after smearing the incubated medium with an indicator, usually a solution of iodine-potassium iodide, and measured in millimeters to obtain the best microbe with the ability to secrete the enzyme for the degradation of the pectin. Usually, the microorganism with the largest diameter after measurement of the clearance zones on the media is taken as the best microbe for producing the enzyme [69]. It is worth mentioning that, this simple microbial technique has been employed in the identification of novel microorganisms with the potential for pectinase and the production of other enzymes [74, 75].
Fermentation is regarded as the primary mode of enzyme production (e.g., pectinase) for industrial uses. This is because the microorganisms employed in the fermentation process act by degrading the more complex substrates into simpler ones with the production of energy [76, 77]. Fermentation is also seen as the use of biological methods to convert sugar to other products anaerobically; with other products like gases, acids, enzymes, and alcohols, also released, and ATP -through substrate-level phosphorylation [44]. As said earlier, Aspergillus niger is the most harnessed microbe in enzyme production. Khan and colleagues [78], report that pectinases are among the few enzymes in demand by industries involved in food processing.
However, the primary fermentation methods for producing enzymes are the solid-state and submerged fermentation methods [79, 80]. Solid-state fermentation (SSF) technique for enzyme production simulates or mimics the original habitats of these microorganisms as in ensiling and composting practices with the microorganisms growing in a small amount of water in or on insoluble materials with the production of useful products [81]. In SSF, the water present is not required beyond the point of saturation [7, 82, 83]. After the production of the enzyme, the next stage is the extraction from the solid medium, or media to recover and purify the required enzyme(s) from solid-state fermentation (SSF) media [84].
In the second fermentation method, the submerged fermentation (SMF), the microorganisms and the nutrients are immersed in water (moisture). This method uses the substrates in liquid form (which flows freely like broths and molasses). The compounds of biological activity are secreted into the fermentation broth. The existence of the nutrients in their free-flowing state aids the rapid utilization of the substrate(s). The need for supplementation with nutrients, like bacteria, is best suited for the SMF medium [85]. Table 3.1 shows some merits of SSF over SMF and its accompanying demerits [44].
Table 1. Some advantages and disadvantages of SSF
Advantages of SSF | Disadvantages of SSF |
a. The lesser water content in substrates used in SSF selectively excludes the growth of bacteria as a contaminant | Microorganisms requiring more moisture content like bacteria may not produce optimally in this method of fermentation |
b. Relatively cheaper substrate preparation compared to submerged fermentation (SMF) | Substrates used in SSF usually need more pre-treatment |
c. A more considerable quantity of enzyme produced compared to SMF, due to the higher growth rate of the microbe(s) makes SSF a preferred choice for enzyme production | Only microorganisms that strive in relatively low moisture environments are favored in SSF [60,85]
|
d. The generated effluents are lower | The frequent need for more concentrations of inoculum in SSF over SMF |
e. The simulation of the natural habitat of the microorganisms used in SSF enhances enzyme production from fungi | The passage of air (aeration) as a result of the higher concentration of solid in SSF poses a challenge as it leads to an extended duration of cultivation as compared to SMF |
f. The enzyme produced is easier to recover from the fermented medium than in SMF | The challenge of expelling metabolic heat is more in SSF than in SMF |
It is, however, necessary to state that submerged fermentation comes with merits such as in instrumentation with regards to the monitoring of parameters like pH, temperature, dissolved oxygen, and sterilization, thereby making this process less difficult to scale up [86, 87]. So, nearly all enzymes of industrial relevance are produced through SSF by the common wild-type microorganisms with the relatively recent emergence in the use of genetically modified microorganisms. The advances in the strategies for the production of pectinase using microorganisms in fermentation have been studied [88].
3.7.1 Microorganisms used in the production of pectinase
A variety of microorganisms are employed in the fermentation process to produce pectinases, such as bacteria and fungi [14, 89]. However, fungi are generally preferred for enzyme production due to the relative safety of their products when consumed by man. These selected microorganisms are Generally Regarded as Safe (GRAS) in comparison to most bacteria; moreover, fungi such as molds and yeasts are closely related to animals as they are eukaryotes in contrast to bacteria [90, 91].
3.7.2 Recovery of the microbial-secreted pectinase
After the fermentation process involving microbial secretion of the pectin enzyme in the medium (usually trapped in the ‘fermented medium’), solvents are usually added to the fermented substrates and agitated to extract the enzyme into the solution and filtered with either Muslin cloth or Whatman filter paper [14]. Thereafter, the filtered enzyme precipitated to concentrate the enzyme and purified based on the application area of the enzyme [14, 84, 92].
The production of crude pectin enzymes has been applied (i.e. without the purification process) [14, 93], as well as with purification [94]. Any application of pectin enzymes with purified pectinases could be an indication that the substrate to be cleaved is the main substrate of interest in the medium. In contrast, for those utilizing crude enzymes, the primary substrate to be cleaved has other accompanying substrates needed to be acted upon by their corresponding enzymes in the crude enzyme mixture; as each enzyme is specific in its action [14, 95]. This, therefore, may account for the reason why not all enzymes undergo purification before their application.
3.7.3 Some factors influencing pectinase production & application
As pectin enzymes are inducible, substrate availability is a crucial factor in their production. However, vital factors such as temperature, pH, inoculum size, and agitation of the fermented substrates [96, 97] along with other factors below and many others equally influence pectinase production, include the following:
a) the substrate type utilized for fermentation
In producing pectinase, the medium used is essential in that each type of pectin enzyme (pectinase) is affected by the type of substrate and the microorganism used in the fermentation process [86, 98, 99]. Production of pectinase needs the corresponding substrate, pectin, for actualization as done for apple pomace [100].
a) the effect of pH
pH influences the production of pectinase as seen with Pseudopestalotiopsis theae [101]. As it affects the growth of the microorganism(s) responsible for the pectinase produced; in unfavorable pH levels, the enzyme could be denatured, thereby significantly reducing its activity. The use of xylano-pectinolytic enzymes by industries engaging in bio-bleaching is reported to produce the enzyme optimally at a pH of 8.5 [102]. Pectinase secreted by Bacillus subtilis was obtained at pH 9.5, and the production of polygalacturonase was produced at acidic pH [103]. So, pH indeed influences the growth of microorganisms and aids its rate of product formation in any medium.
c) Effect of temperature on the production of pectinase
The protein attribute of enzymes cannot be overlooked in considering temperature, as high temperature denatures enzymes, thereby negatively affecting their activity. Studies undertaken concerning optimizing the effect of temperature on pectinase production have shown that temperature affects pectinase production. The temperature, 30oC with Saccharomyces cerevisiae (ATCC 52712), also Pseudopestalotiopsis theae showed the best activity for pectinase production at 30oC and Aspergillus niger, and 37oC with Bacillus subtilis were the optimal temperatures obtained beyond which there was a decline in pectinase activity [14, 97, 101, 104]. Polygalacturonase's best activity from the mold was obtained at 50oC and 40oC to 60oC for yeasts in another study [105], as 25oC was reported for another strain of Saccharomyces cerevisiae by Magdy [106]. So, differences in strains of microorganisms could lead to differences in the optimum temperature for the best activity.
d) Effect of fermentation time and substrate composition on pectinase production
Hussain and colleagues [104] reported the production of pectinase on the 4th day using the conventional approach of one factor at a time (OFAT). The 7th day was for endoglucanase production with the best activity using Aspergillus niger, Aspergillus flavus, and Penicillium atrovenetum.
Agrowastes like the peels of pineapple and orange as well as sawdust, wheat bran, and sugar cane pulps were also used as substrates for the production of pectinase with strains of Aspergillus clavatus, Aspergillus niger, Fusarium sp., Penicillium chrysogenum, and Trichoderma sp [107].
e) Effect of solvents on pectinase extraction
Some extraction solvents like distilled water have been used in the recovery of some enzymes from the fermentation medium, buffer, and salts among many other extraction solvents [108, 109]. The rationale for the use of solvent for extraction is that it aids in the splitting of the bond between proteins and carbohydrates of such substrates, thereby, releasing the ‘trapped enzyme’. The extraction solvent for the recovery of the enzyme from the fermented substrate is essential. Where the solvent volume is too much, it gives rise to a dilute solution with reduced enzymatic activity [110]. In another report, in cases of reduced volume of buffer, decreased enzyme activity is obtained with the same report advocating for more extraction solvent volume [111]. So, researchers need to optimize the actual volume of extraction solvent necessary for the best activity of such enzyme as a reduced volume of solvent leads to the insufficiency of the solvent to permeate the solid fermented mass in cases of SSF.
f) Effect of ammonium sulfate precipitation for practical application of enzyme
The effect of ammonium sulfate salt primarily precipitates the protein from the extract [99]. The groups on the molecules of proteins with charges are stabilized in the concentration of low salts. This process increases the solubility of such proteins; hence, resulting in a phenomenon referred to as salting-in. In some situations, increased salt addition leads to insoluble protein due to a reduction in water, making the proteins precipitate, a phenomenon referred to as salting out in response to excess salt.
A study by Joshi and colleagues showed that in purifying PME (pectin methyl lyase), using 20 - 80% NH4(SO4)2, an increase in the salt concentration further led to an increase in enzyme activity with about 160.6% increase in 80% (i.e., 21.50 for 80%) of the salt in comparison to 8.25 for 0%. In the same study, however, the soluble content of protein was reduced from 62 mg/ml to 21 mg/ml, with an 80% ammonium sulfate concentration of the protein in its crude state [112]. The use of ammonium sulfate has also increased by 30% of the yield of pectinase [113].
Dialysis with ammonium sulfate precipitation has proven to increase the purification of the crude enzyme from orange peel [114].
Apart from using ammonium sulfate for partial purification through precipitation of crude protein, ethanol, and ethylene glycol are reportedly used, as chilled acetone or ethanol are added slowly to the enzyme solution and incubated in an ice-salt bath with continuous stirring [100, 115-117].
g) Effect of enzyme dosage for effective extraction of fruit juice
In the extraction of apple juice, 2.5% was obtained for the best activity, though apple and pear juice clarifications were best achieved with 1.0 and 0.5%, respectively [112]. The scanning electron microscope found that pectinases used to treat fruits led to the hydrolysis of the middle lamella (made up of xylan and pectin), thereby releasing more quantity of juice [117].
h) Effect of metal ions and inhibitors
Enzymes are known to have active sites that participate in the reaction process. However, on each enzyme's active sites are functional groups or groups that aid catalysis, though the substrate changes in the orientation relative to the functional group on the active site [34].
For catalytic stability in the conformation of the enzyme to be maintained, metal ions could accept or give out electrons, thereby restricting mainly the desired reaction process to take place as it limits other undesired reactions; this process keeps hold of the substrate and enzyme bond, before the product formation [84, 118]. Most enzymes with pectin enzymes inclusive are known to be metalloenzymes. Hence, they need metal ions such as Ca2+, Mn2+, Fe2+, and Mg2+, among many other ions, to increase their activity [119]. However, some elements reduce the interaction of the substrate with the enzyme’s active sites, and these are given the term inhibitors [84]. Interestingly, a systematic review in the last decade on the production and application of pectinases and the investigation into both pectin and pectinase activity using flavedo and albedo citrus fruit peels have been investigated [15, 16].
4. Conclusions
Pectinases are produced from cheap raw materials such as agro-wastes (through fermentation). The production of pectinase is ‘hinged’ on the substrate, pectin; which is a component in the cell wall of plant materials and is also known as the inducer. The classification of pectin shows that pectins are distinct in their properties hence accounting for the differences in their pectinase production tendencies and the relative modes of action in a catalytic process; since the alteration of pectin structure in fruits by pectin enzymes leads to the breaking of the bonds. Though animals could be used to produce pectin enzymes (pectinases), microorganisms through fermentation have proven over time from findings to have a shorter duration and ease of harvest of the enzyme among other benefits, compared to producing pectinases using animal sources. Hence, their predominant utilization in the production of pectinases. A number of factors have been considered to aid pectinase production; ranging from the type of pectin substrate used, pH, temperature, fermentation duration, extraction solvents, metal ions, the purification method used; and, the dosage used for the enzyme application purpose. The classification of pectinases and modes of action is indicative of the different pectin enzymes produced during fermentation and their corresponding application areas. Purification of pectin enzymes was not given much credence in this study because, studies have emerged on the utilization of crude pectinase for application purposes; such as, in the extraction and clarification of fruit juices.
4.1 Related current challenges
The existence of many microorganisms (uncultured) has limited their application for use in the fermentation process for pectinase production. The fungi ‘kingdom’ is common knowledge in the scientific world; however, with the increase in technology comes the emergence and updating of the data of the 'uncultured microorganisms.' It is interesting to know that this and other microorganisms are now determined using deoxyribonucleic acid (DNA) sequencing techniques [75]. Hence, there is a need for simple and relatively cheaper technique for the cultivation and identification of the many ‘uncultured microorganisms’ and the investigation of their pectinase production potentials.
4.2 Next steps for future research and approaches
Owing to the notion that the present commercial extraction procedure destroys the pectin (limiting the potential product uses) and is hazardous to the environment, hence alternative pectin extraction processes are urgently needed. The steps for future research are expected to be more on the use of Microwave-Assisted Extraction to provide a sustainable method of extracting pectin from a variety of food wastes and agricultural leftovers [120]. Additionally, deep eutectic solvent (DES) had a larger pectin yield, a lower degree of esterification, and a slightly different monosaccharide composition than acid extraction (AE) and ultrasonic-assisted extraction (UAE), according to the testing data. Infrared spectroscopy and scanning electron microscopy studies revealed that DES had a fine microstructure and a coarser surface, but the basic chemical structure of DES remained unchanged; the results showed that a green source of pectin extraction with a high pectin yield and good performance is possible with DES [121,122]. Hence, there is a need for future research to optimize the conditions for the improved use of natural eutectic solvents, microwave-assisted extraction and ultrasonic-assisted extraction for pectin (extraction). Harnessing these technologies may reduce the adverse effects on the environment occasioned by the use of strong acids due to pectin extraction [123].
Authors’ contributions
Conceptualization and writing of original draft preparation, G.D.A.; Editing and supervision, F.A.O.
Acknowledgements
We acknowledge the authors whose studies provided the basis for this review.
Funding
This research received no specific grant from any funding agency “(the public, commercial, or not-for-profit sectors)”.
Availability of data and materials
All data will be made available on request according to the journal policy
Conflicts of interest
The authors declare no conflict of interest
References
1.
Micheletti
C.; Medori M.C.; Iaconelli A.; Aquilanti B.; Matera G.; Bertelli M. (2023).
Effects of carob extract on the intestinal microbiome and glucose metabolism: a
systematic review and meta-analysis. Clin. Ter. 2023, 174(Suppl 2(6)), 169-172.
2.
Pedrolli
D.B.; Alexandre C.M.; Eleni G.; Eleonora C.C. Pectin
and pectinases: production, characterization and industrial application of
microbial pectinolytic enzymes. The Open Biotechnol. J. 2009, 3, 9-18. https://doi.org/10.2174/1874070700903010009
3.
Su D.L.; Li P.J.; Quek S.Y.; Huang
Z.Q.; Yuan Y.J.; Li G.Y.; Shan Y. Efficient extraction and characterization of
pectin from orange peel by a combined surfactant and microwave-assisted
process. Food Chem. 2019, 286, 1-7. https://doi.org/10.1016/j.foodchem.2019.01.200
4.
Ike
C.C.; Ezeibe C.C.; Anijiofor S.C.; Nik-Daud N.N. Solid waste management in
Nigeria. Problems, prospects, and policies. J. Solid Waste Technol Manag. 2018, 44(2), 163-172. https://doi.org/10.5276/jswtm.2018.163
5.
Freitas C.M.P.; Coimbra J.S.R.; Souza V.G.L.; Sousa R.C.S.
Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical
Industry: A Review. Coatings. 2021, 11,
922. https://doi.org/10.3390/coatings11080922
6.
Wang W.; Chen W.; Zou M.; Lv R.; Wang D.; Hou F.; Feng H.; Ma
X.; Zhong J.; Ding T.; et al. Applications of power ultrasound in oriented
modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. https://doi.org/10.1016/j.jfoodeng.2018.04.016
7.
Oumer,O.J.
Pectinase: substrate, production and their biotechnological applications. Int.
J. Environ. Agric. Biotechnol. (IJEAB). 2017, 2(3), 1007-1014. https://doi.org/10.22161/ijeab/2.3.1
8.
Chen J.; Niu X.; Dai T.; Hua H.; Feng S.; Liu C.; McClements
D.J.; Liang R. Amino acid-amidated pectin: preparation and
characterization. Food Chem. 2020, 309,
125768. https://doi.org/10.1016/ j.foodchem.2019.125768
9.
Leijdekkers A.; Bink J.; Geutjes S.; Schols H.; Gruppen H.
Enzymatic saccharification of sugar beet pulp for the production of
galacturonic acid and arabinose; a study on the impact of the formation of
recalcitrant oligosaccharides. Bioresour. Technol. 2013, 128, 518–525. https://doi.org/10.1016/
j.biortech.2012.10.126
10.
Zhang S.; Hu H.; Wang L.; Liu F.; Pan S. Preparation and
prebiotic potential of pectin oligosaccharides obtained from citrus peel
pectin. Food
Chem. 2018, 244,
232–237. https://doi.org/10.1016/j.foodchem.2017.10.071
11.
Belkheiri A.; Forouhar
A.; Ursu A.; Dubessay P.; Pierre G.; Delattre C.; Djelveh G.; Abdelkafi S.;
Hamdami N.; Michaud P. Extraction, Characterization, and applications of
pectins from plant by-products. Appl. Sci. 2021, 11, 6596. https://doi.org/10.3390/app11146596
12.
Capel F.; Nicolai T.; Durand D.; Boulenguer P.; Langendorff
V. Calcium and acid-induced gelation of (amidated) low methoxyl pectin. Food Hydrocoll. 2006, 20, 901–907. https://doi.org/10.1016/j.foodhyd.2005.09.004
13.
World
Population Data Sheet, 2023. Available online:
https://www.prb.org/wp-content/uploads/ 2023/12/2023-World-Population-Data-Sheet-Booklet.pdf
(accessed on 28 December 2023).
15.
Ametefe G.D.; Lemo A.O.; Ugboko H.U.; Iweala E.E.J.;
Chinedu S.N. Pectinase production and application in the last decade: a
systemic review. In: Ayeni, A.O., Sanni, S.E., Oranusi, S.U. (eds) Bioenergy
and Biochemical Processing Technologies. Green Energy and Technology: Springer,
Cham. (2022). https://doi.org/10.1007/978-3-030-96721-5_12
16.
Ametefe G.D.; Iheagwam F.N.; Fashola F.; Ibidapo O.I.;
Iweala E.E.J.; Chinedu S.N. Comparison of pectinase activity in the flavedo and
albedo of citrus and Thaumatococcus daniellii fruits. In:
Ayeni, A.O., Sanni, S.E., Oranusi, S.U. (eds) Bioenergy and Biochemical
Processing Technologies. Green Energy and Technology: Springer, Cham. (2022). https://doi.org/10.1007/978-3-030-96721-5_17
17.
Kumar
M.; Tomar M.; Saurabh V.; Mahajan T.; Punia S.; Contreras M.; Rudra S.G.; Kaur
C.; Kennedy J.F. Emerging trends in pectin extraction and its anti-microbial
functionalization using natural bio-actives for application in food packaging.
Trends Food Sci. Technol. 2020, 105, 223-237. https://doi.org/10.1016/j.tifs.2020.09.009
18. Maskey B.;
Dhakal D.; Pradhananga M.; Shrestha, M.
Extraction and process optimization of bael fruit pectin. Food Sci. Nutr. 2018, 6, 1927-1932. https://doi.org/10.1002/fsn3.761
19.
McKay
S.; Oranje P.; Helin J.; Koek J.H.; Kreijveld E.; Van den Abbeele P.; Pohl U.;
Bothe G.; Tzoumaki M.; Aparicio-Vergara M.; Mercenier A.; Schols. H.
Development of an Affordable, Sustainable, and Efficacious Plant-Based
Immunomodulatory Food Ingredient Based on Bell Pepper or Carrot RG-I Pectic
Polysaccharides. Nutrients. 2021, 13, 963. https://doi.org/10.3390/nu13030963
20.
Tan J.; Hua X.; Liu J.; Wang M.; Liu
Y.; Yang R.; Cao Y. Extraction of sunflower head pectin with superfine grinding
pretreatment. Food Chem. 2020, 320, 126631. https://doi.org/10.1016/j.foodchem.2020.126631
21.
Rehman H.; Baloch
A.H.; Asif N.M. Pectinase: immobilization and
applications. a review. Trends Pept. Protein Sci. 2021, 6,
el.
22. Zouambia Y.; Ettoumi K.Y.; Krea M.;
Moulai-Mostefa N. A new approach for pectin extraction: electromagnetic induction
heating. Arab. J. Chem. 2017, 10, 480-487. https://doi.org/10.1016/j.arabjc.2014.11.011
23.
Harholt
J., Suttangkakul A., Scheller H.V. Biosynthesis of pectin. Plant Physiology. 2010,
153, 384-395. https://doi.org/10.1104/pp.110.156588
24.
Verma
H.; Narnoliya L.K.; Jadaun J.S. Pectinase: A useful tool in fruit processing
industries. Nutr. Food Sci. 2018,
5(5), 555673.
25.
Zhang Q.; Dai W.; Jin X.;
Li J. Calcium chloride and 1-methylcyclopropene treatments delay
postharvest and reduce decay of New Queen melon. Sci. Rep. 2019, 9(1), 13563. https://doi.org/10.1038/s41598-019-49820-8
26.
Shinga
M.H.; Fawole O.A. Opuntia ficus indica mucilage coatings regulate cell wall softening
enzymes and delay the ripening of banana fruit stored at retail conditions. Int.
J. Biol. Macromol. 2023, 245, 125550, ISSN 0141-8130, https://doi.org/10.1016/j.ijbiomac.2023.125550.
27.
Wang
W.; Yu J.; Du M.; Wang J.; Hu D. Basic helix-loop-helix (bHLH) transcription
factor MdbHLH3 negatively affects the storage performance of postharvest apple
fruit. Hort. Plant J. 2022, 8(6), 700-712. https://doi.org/10.1016/j.hpj.2022.08.005
28.
Li X.; Su Q.; Jia R. J.; Wang Z.; Fu J.; Guo
J.H.; Yang H.J.; Zhao Z. Comparison of cell wall changes of two different types
of apple cultivars during fruit development and ripening. J. Int. Agric. 22(9),
2023, 2705-2718, ISSN 2095-3119. https://doi.org/10.1016/j.jia.2023.07.019
29.
Alkorta
I.; Gabirsu C.; Lhama M.J.; Serra J.L. Industrial applications of pectic
enzymes: a review. Proc. Biochem. 1998, 33,
21-28. https://doi.org/10.1016/s0032-9592(97)00046-0
30.
Broxterman
S.E.; Schols H.A. Interactions between pectin and cellulose in primary cell
walls. Carbohydr. Polym. 2018, 192, 263-272. https://doi.org/10.1016/j.carbpol.2018.03.070
31.
Jayani
R.S.; Saxena S.; Gupta R. Microbial pectinolytic enzymes: A review. Proc.
Biochem. 2005, 40, 2931-2944. https://doi.org/10.1016/j.procbio.2005.03.026
32.
Muller-Maatsch
J.; Bencivenni M.; Caligiani A.; Tedeschi T.; Bruggeman G.; Bosch M.; ...
Sforza S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37-45. https://doi.org/10.1016/j.foodchem.2016.01.012
33.
Chapman
J.; Ismail A.E.; Dinu C.Z. Industrial applications of enzymes: recent advances,
techniques, and outlooks. Catalysts. 2018,
8, 238. https://doi.org/10.3390/catal8060238
34.
Vitolo
M. Brief review on enzyme activity. World
J. Pharm. Res. 2020, 9(2),
60-76.
35. Martens E.C.;
Neumann M.; Desai M.S. Interactions of commensal and pathogenic microorganisms
with the intestinal mucosal barrier. Nat.
Rev. 2018, 16, 457-470. https://doi.org/10.1038/s41579-018-0036-x
36. Thursby E.; Juge N. Introduction to the
human gut microbiota. Biochem J. 2017,
474, 1823-1836. https://doi.org/10.1042/bcj20160510
37. Jin X.; Song
J.; Song G.Q. Bioethanol production from rice straw through an enzymatic route
mediated by enzymes developed in-house from Aspergillus fumigatus. Energy. 2020, 190, 1-10. https://doi.org/10.1016/j.energy.2019.116395
38.
Prasoulas
G.; Gentikis A.; Konti A.; Kalantzi S.; Kekos D.; Mamma D. Bioethanol
production from food waste applying the multienzyme system produced on-site by Fusarium
oxysporum F3 and mixed microbial cultures. Ferment. 2020, 6, 39-50.
https://doi.org/10.3390/fermentation6020039
39. Mazurenko S.;
Prokop Z.; Damborsky J. Machine learning in enzyme engineering. ACS Catalysis. 2020, 10, 120-1223.
40.
Marwaha
S.S.; Arora J.K. Food processing: biotechnological applications. 1st ed.,
Asiatech Publisher Inc. New Delhi. 2000.
41. Pham J.V.;
Yilma M.A.; Feliz A.; Majid M.T.; Maffetone N.; Walker J.R.; Yoon, Y.Y. A review of the microbial production of
bioactive natural products and biologics. Front Microbiol. 2019, 10,
1-27.
42.
Suryam
A.; Rafiyuddin M.; Vasu K.; Singara M.A. Production of endo pectolytic enzymes
by six fruit rot fungi and their use in fruit juice extraction and
clarification. Int. J. Recent Sci.
Res. 2019, 10(3C),
31322-31330.
43.
Tabssum
F.; Ali S.S. Screening of pectinase
producing gram-positive bacteria: Isolation and characterization. Punjab Univ. J. Zool. 2018, 33(1), 11-15. https://doi.org/10.17582/pujz/2018.33.1.11.15
45.
Cardoso
F.S.N.; Koblitz M.G.B.; Oritz M.G.B.; Carvalho J.L.V.; Carvalho L.M.J. Study of
the parameters used in the encapsulation of commercial pectinase in calcium
alginate and its effect on its catalytic activity. Food Sci. Technol. 2019, 39(1),
247-252. https://doi.org/10.1590/fst.31518
46.
Li
K.; Meng K.; Pan X.; Ma R.; Yang P.; Huang H.; … Su X. Two thermophilic fungal
pectinases from Neosartorya fischeri P1: gene clone expression and
biochemical characterization. J. Mol.
Catal. B: Enzym. 2015, 118, 70-78.
https://doi.org/10.1016/j.molcatb.2015.05.005
47.
Pastore
G.M. FEA/UNICAMP, Production and uses of enzymes in food processing. (2001).
www.anbio.org.br/english/work8h43.htm accessed on 18/12/2017.
48.
Lang
C.; Dörnenburg H. Perspectives in the biological function and the technological
application of polygalacturonases. Appl. Microbiol. Biotechnol. 2000, 53, 366-375. https://doi.org/10.1007/s002530051628
49. Hours R.A.; Sakai T. Growth and
protopectinase production of Aspergillus awamori in solid-state culture
at different acidities. J. Ferment
Bioeng. 1994, 78(6), 426-430. https://doi.org/10.1016/0922-338x(94)90041-8
50. Wen B.; Zhang F.; Wu X.; Li H.
Characterization of the tomato (Solanum lycopersicum) pectin
methylesterases: evolution, activity of isoforms and expression during fruit
ripening. Front Plant Sci. 2020,
11, 238, 1-17. https://doi.org/10.3389/fpls.2020.00238
51.
Gholizadeh
A. Pectin methylesterase activity of plant DUF538 protein superfamily. Physiol. Mol. Biol. Plants. 2020, 26(4), 829-839. https://doi.org/10.1007/s12298-020-00763-9
52.
Yuan
Y.; Zhang X.Y.; Zhao Y.; Zhang H.; Zhou Y.F.; Gao J. A novel PL9 pectate lyase
from Paenibacillus polymyza KF-1: cloning, expression and its
application in pectin degradation. Int.
J. Mol. Sci. 2019, 20, 1
-17. https://doi.org/10.3390/ijms20123060
53.
Zhao
Y.; Yuan Y.; Zhang X.; Li Y.; Li Q.; Zhou Y.; Gao J. Screening of a novel
polysaccharide lyase family 10 pectate; lyase from Paenibacillus polymyxa KF-1:
cloning, expression, and characterization. Molecules. 2018, 23, 2774-2788.
https://doi.org/10.3390/molecules23112774
54. Maciel M.;
Ottoni C.; Santos C.; Lima N.; Moreira K.; Souza-Motta C. Production of
polygalacturonases by Aspergillus section Nigri strains in a fixed
bed reactor. Molecules. 2013, 18, 1660-1671. https://doi.org/10.3390/molecules18021660
55.
Bohlmann
H.; Sobczak M. The plant cell wall in the feeding sites of cyst nematodes. Front Plant Sci. 2014, 5, 89. https://doi.org/10.3389/fpls.2014.00089
56.
Holbeing
J.; Grundler F.M.W.; Siddique S. Plant basal resistance to nematodes: an
update. J. Exp Bot. 2016, 67, 7, 2049-2061. https://doi.org/10.1093/jxb/erw005
57.
Munir
N.; Javaid A.M.; Haidri S.H. Production, purification and characterisation of
endopolygalacturonase Bacillus subtillus. Biochem. Anal. Biochem. 2015, 4(3), 1-12. https://doi.org/10.4172/2161-1009.1000181
58.
Ntalli
N.; Adamski Z.; Doula M.; Monokrousos N. Nematicidal amendments and soil
remediation. Plants. 2020, 9, 429-449. https://doi.org/10.3390/plants9040429
59.
Flodrova
D.; Garajova S.; Malovikova A.; Mislovicova D.; Omelkova J.; Stralilova E.
Oligogalacturonate hydrolase with unique substrate preference from the pulp
parsley root. Biologia. 2009, 64, 2, 228–234. https://doi.org/10.2478/s11756-009-0038-2
60. Oumer O.J.; Abete D. Comparative
studies of pectinase production by Bacillus subtilis strain Btk 27 in
submerged and solid-state fermentations. Bio.
Med. Res. Int. 2018, Article ID 1514795, https://doi.org/10.1155/2018/1514795 .
62. Sitanggang A.B.; Sinaga W.S.L.; Wie F.;
Fernando F.; Krusong W. Enhanced antioxidant activity of okara through
solid-state fermentation of GRAS fungi. Food
Sci. Technol. 2020, 40(1),
178-186. https://doi.org/10.1590/fst.37218
63.
Kalb V.; Seewald T.; Hofmann T.;
Granvogl M. Correction to the role of endogenous enzymes during malting of barley and wheat varieties in the
mitigation of styrene in wheat beer. J.
Agric. Food Chem. 2021, 69, 1156. https://doi.org/10.1021/acs.jafc.0c07142
64.
Kermani
Z.J.; Shpigelman A.; Houben K.; Guezendam B.; Loey A.M.V.; Hendrickx M.E. Study
of mango endogenous pectinases as a tool to an engineer mango puree
consistency. Food Chem. 2015, 172, 272-282. https://doi.org/10.1016/j.foodchem.2014.09.077
65.
Othman
A.S. Isolation and microbiological identification of bacterial contaminants in
food and household surfaces: how to deal safely. Egypt Pharm J. 2015, 14,
50-55. https://doi.org/10.4103/1687-4315.154720
66.
Carrazco-Palafox
J.; Rivera-Chavira B.E.; Ramirez-Baca N.; Manzanares-Papayanopoulos L.I.;
Nevarez-Moorillon G.V. Improved method
for qualitative screening of lipolytic bacterial strains. MethodsX. 2018, 5, 68-74. https://doi.org/10.1016/j.mex.2018.01.004
67.
Manal
S.S.; Sahar S.M.; Manal G.M.; Mohsen M.A.; Osama H.E.S. Purification and
kinetics of pectinase production from Paenibacillus lactis NRC1 locally
isolated from Egyptian mangrove habitat. Der
Pahrma Chem. 2016, 8(9),
150–159.
68.
Ramnath
L.; Sithole B.; Goviden R. Identification of lipolytic enzymes from bacteria
indigenous to Eucalyptus wood species for application in the pulping
industry. Biotechnol. Rep. 2017,
15, 114-124. https://doi.org/10.1016/j.btre.2017.07.004
69.
Takci
A.A.M.; Turkmen F.U. Extracellular pectinase production and purification from
newly isolated Bacillus subtilis strain. Int. J. Food Prop. 2016, 19,
2443-2450. https://doi.org/10.1080/10942912.
2015.1123270
70.
Ametefe
G.D., Lemo A.O., Orji F.A., Kolawole L.A., Iweala E.E.J., Chinedu S.N. (2021).
Comparison of optimal fungal pectinase activites using the Box-Behnken Design.
Trop. J. Nat. Prod. Res. 5(9), 1656-1664. https://doi.org/10.26538/tjnpr/v5i9.21
71.
Adeyemi
A.O.; Efunwole T.K.; Olanbiwoninu A.A. Comparative bacteriological analysis of
stored borehole water sources in Oyo town, Oyo State, Nigeria. Afr. J. Microbiol Res. 2020, 14, 1, 32-41. https://doi.org/10.5897/ajmr2019.9129
72.
Chinedu
S.N.; Nwinyi O.C.; Okochi V.I. Growth and cellulase activity of wild type Aspergillus
niger ANL301 in different carbon sources. Can. J. Pure Appl. Sci. 2008, 2(2), 357–362.
73.
Poveda
G.; Gil-Duran C.; Vaca I.; Levican G.; Chavez R. Cold-active pectinolytic
activity produced by filamentous fungi associated with Antarctic marine
sponges. Biol. Res. 2018, 51, 28-32. https://doi.org/10.1186/s40659-018-0177-4
74.
Bibi N.; Ali S.; Tabassum, R. Isolation
and identification of novel indigenous bacteria strain as a low-cost pectinase
source. Braz. Arch. Biol. Technol. 2018, 61, e18160653. https://doi.org/10.1590/1678-4324-2018160653
75.
Shrestha S.; Khatiawada J.R.; Zhang X.;
Chio C.; Kognou A.L.M.; Chen F.; Han S.; Chen X.; Qin W. Screening and
molecular identification of novel pectinolytic bacteria from forest soil.
Ferment. 2021, 7, 40. https://doi.org/10.3390/fermentation7010040
76. Renge V.C.; Khedkar S.V.; Nikita
R.N. Enzyme synthesis by fermentation method: A review. Sci. Revs. Chem. Commun. 2012, 2(4), 585-590.
77.
Ward
O.P. Fermentation Biotechnology: Principles, processes and products, 2nd ed.;
John Wiley and Sons: New York, USA, 1992.
78.
Khan
A.; Sahay S.; Rai N. Production and optimization of
pectinase enzyme using Aspergillus niger in
solid state fermentation. Res. Biotechnol. 2012, 3(3), 19-25.
79.
Rangarajan
V.; Rajasekeran M.; Ravichandran R.; Sriganesh K.; Vaitheeswaran V. Pectinase
production from orange peel extract and dried orange peel solid as substrates
using Aspergillus niger. Int. J.
Biotechnol. Biochem. 2010, 6, 445-453.
80.
Soccol
C.R.; Larroche C. Application of tropical agro-industrial residues as substrate
for SSF. Sringer Science +Business Media, LLC, Spring Street, New York.2008.
81.
Pitol
L.O.; Biz A.; Mallmann E.; Krieger N.; Mitchell D.A. Production of pectinases
by solid-state fermentation in a pilot scale packed bed bioreactor. Chem. Eng. J. 2016, 283, 1009-1018. https://doi.org/10.1016/j.cej.2015.08.046
82.
Manan
M.A.; Webb C. Modern microbial solid-state fermentation technology for future
biorefineries for the production of added-value products. Biofuel Res. J. 2017, 4, 730–740. https://doi.org/10.18331/brj2017.4.4.5
83.
Manan
M.A.; Webb C. Design aspects of solid-state fermentation as applied to
microbial bioprocessing. J. Appl. Biotech.
& Bioproc. 2017, 4, 1,
91. https://doi.org/10.15406/jabb.2017.04.00094
84.
Okonji
R.E.; Itakorode B.O.; Ovumedia J.O.; Adedeji O.S.
Purification and biochemical characterization of pectinase produced by Aspergillus fumigatus isolated from soil
of decomposing plant materials. J.
Appl. Biol. Biotech. 2019, 7(3),
1-8. https://doi.org/10.7324/jabb.2019.70301
85.
Subramaniyam
R.; Vimala R. Solid state and submerged fermentation for the production of
bioactive substances: A comparative study. Review article. Int. J. Sci. Nature.
2012, 3(3), 480-486.
86.
Farinas
C.S. Developments in solid-state fermentation for the production of
biomass-degrading enzymes for the bioenergy sector. Renew Sustain. Energy Rev. 2015, 52, 179-188. https://doi.org/10.1016/j.rser.2015.07.092
87.
Matthew
J.J.; Vazhacharickal P.J.; Sajeshkumar N.; Ashokan A. Amylase production by Aspergillus
niger through submerged fermentation using starchy food byproducts as
substrate. Int. J. Herb. Med.
2016, 4(6), 34-40.
88.
Amin
F.; Bhatti H.N.; Bilal M. Recent advances in the production strategies of
microbial pectinases- A review. Int. J. Biol. Macromol. 2019, 122, 1017-1026. https://doi.org/10.1016/j.ijbiomac.2018.09.048
89. Hassan R.; Aktar N.; Kabir S.T.; Honi
U.; Halim A.; Islam R.; ... Islam M.S. Pectinolytic bacteria consortia reduce
jute retting period and improve fibre quality. Sci. Rep. 2020, 10, 1-10.
https://doi.org/10.1038/s41598-020-61898-z
90.
Sewalt
V.; Shanahan D., Gregg L.; Marta J.L.; Corrillo R. The Generally Recognized as
Safe (GRAS) Process for industrial microbial enzymes. Ind. Biotechnol. 2016,
12(5), 295-302. https://doi.org/10.1089/ind.2016.0011
91.
Naranjo-Ortiz
M.A.; Gabaldon T. Fungal evolution: diversity, taxonomy and phylogeny of the
fungi. Biol. Rev. 2019, 94, 2101-2137. https://doi.org/10.1111/brv.12550
92.
Bharadwaj P.S. Extraction and purification of
peroxidase enzyme from sweet potato. The
Pharma Innov. J. 2019, 8(9),
418-422.
93.
Shariq
M.; Sohail M. Citrus limetta peels: a promising substrate for the
production of multienzyme preparation from a yeast's consortium. Bioresour. Bioproc. 2019, 6, 43-58. https://doi.org/10.1186/s40643-019-0278-0
94.
Almulaiky
Y.Q.; Albishri A.A.; Khalil N.M.; Algamal Y.; Aldhahri M.; Al-Harbi S.A.; ...
Alhadi R. Polygalactutonase by Aspergillus niger using seaweed waste
under submerged fermentation: production, purification and characterization. J. Sci. Technical Res. 2020, 25(4), 19416-19422. https://doi.org/10.26717/bjstr.2020.25.004249
95.
Ajayi
A.A.; Osunkoya F.A.; Peter-Albert C.F.; Olasehinde G.I. Clarification of apple
juice with laboratory-based-pectinase obtained from deterioration of apple (Malus
domestica) by Aspergillus niger. Int. J. Adv. Biotechnol. Res. 2014, 5(2), 134-140.
96.
Apoorvi
C.; Vuppu S. Microbial Derived Pectinases: A Review. IOSR-JPBS, 2012, 2(2),
1-5.
97.
Sonia
A.; Saurabh S.D.; Bindu B.; Mandhan R.P.; Jitender S. Pectinase production by Bacillus subtilis and its potential
application in biopreparation of cotton and micropoly fabric. Proc. Biochem.
2009, 44(5), 521-526. https://doi.org/10.1016/j.procbio.2009.01.003
98.
Rehman
U.H.; Siddique N.V.; Aman A.; Nawaz M.A.; Baloch A.H.; Qader S.A.U. Morphological and molecular based identification of
pectinase producing Bacillus licheniformis from rotten vegetable. J. Gen.c
Eng. Biotechnol. 13(2), 2015, 139-144. https://doi.org/10.1016/j.jgeb.2015.07.004
99.
Ismail
A.S.; Abo-Elmagd H.I.; Housseiny M.M. A safe potential juice clarifying
pectinase from Trichoderme viridae EF-8
utilizing Egyptian onion skins. J. Gen.c Eng. Biotechnol. 14(2), 2016, 153-159.
https://doi.org/10.1016/j.jgeb.2016.05.001
100. Pathania S.; Sharma N.; Handa S.
Utilization of horticultural waste (apple pomace) for multiple carbohydrase
production from Rhizopus delmar F2
under solid state fermentation. J. Gen.c Eng. Biotechnol. 16(1), 2018, 181-189.
https://doi.org/10.1016/j.jgeb.2017.10.013
101. Sopalun K.; Iamtham S. Isolation and
screening of extracellular enzymatic activity of endophytic fungi isolated from
Thai orchids. South Afr. J. Bot. 134, 2020, 273-279. https://doi.org/10.1016/j.sajb.2020.02.005
102. Amanjot K.; Ritu M.; Avtar S.; Gaurav
G.; Jitender S. Application of cellulase-free xylano-pectinolytic enzymes from
the bacterial isolate in bio-bleaching of kraft pulp. Bioresour. Technol. 2010,
101(3), 9150-9155. https://doi.org/10.1016/j.biortech.2010.07.020
103. Suneetha V.; Zaved A.K. Actinomycetes:
Sources for Soil Enzymes, Shukla, G. and Varma, A. (ed), Soil Enzymology, Soil
Biology-22, (Springer-Verlag Berlin Heidelberg). 2010 p 259-269
104. Hussain K.; Wajid A.; Babar M.E.; Anwar
Z.; Farooqi S.; Siddiqa A.; ... Iqbal J. Production and optimization of
pectinase from pomelo by Aspergillus niger through solid state
fermentation. Ann. Life Sci. 2019,
6, 21-41.
105. Ernesto F.T.; Tania
V.S.; Gustavo V.G. Production of hydrolytic depolymerizing pectinases. Food Technol. Biotechnol. 2006, 44(2), 221-227.
106. Magdy M.A. Effective technological
pectinase and cellulase by Saccharomyces
cerevisiae utilizing food wastes for citric acid production. Life Sci. J.
2011, 8(2), 305-313.
107. Okafor U.A.; Okochi V.I.; Chinedu S.N.;
Ebuehi O.A.T.; Onygeme-Okerenta B.M. Pectinolytic activity of wild-type
filamentous fungi fermented on agro-wastes. Afr. J. Microbiol. Res. 2010, 4(24), 2729-2734.
108. Linde G.A.; Magagnin
G.; Costa J.A.V.; Bertolin, T.E.; Colauto, N.B. Column bioreactor use for
optimization of pectinase production in solid-state cultivation. Braz. J.
Microbiol. 2007, 38, 557-562. https://doi.org/10.1590/s1517-83822007000300033
109. Patil N.P.; Chaudhari B.L. Production
and purification of pectinase by a soil isolate Penicillium sp. and
search for better agro-residue for its SSF. Recent Res. Sci. Technol. 2010, 2(7), 36-42.
110. Aikat K.; Bhattacharyya B.C. Protease
extraction in solid-state fermentation of wheat bran by a local strain of Rhizopus oryzae and growth studies by
the soft gel technique. Proc. Biochem. 2000, 35, 907-914. https://doi.org/10.1016/s0032-9592(99)00148-x
111. Ahmed S.A.; Mostafa F.A. Utilization of
orange baggase and molokhia stalk for the production of pectinase enzyme. Braz.
J. Chem. Eng. 2013, 30(3), 449-456. https://doi.org/10.1590/s0104-66322013000300003
112. Josh V.K.; Parmar
M.; Rana N. Purification and characterization of pectinase produced from
Apple pomace and evaluation of its efficacy in fruit juice extraction and
clarification. Indian J. Nat. Prod. Resour. 2008, 2(2), 189 - 197. https://doi.org/10.1016/j.jbiotec.2008.07.1866
113. Singh S.A.; Ramakrishna M.; Rao A.G.
Optimization of downstream processing parameters for the recovery of pectinase
from the fermented bran of Aspergillus
carbonarius. Proc. Biochem. 1999, 35, 411-417. https://doi.org/10.1016/s0032-9592(99)00089-8
114. Ezugwu A.L.; Ezike
T.C.; Ibeawuchi A.N.; Nsude C.A.; Udenwobele D.I.; Eze S.O.O.; … Chilaka F.C. Comparative Studies on
pectinases obtained from Aspergillusfumigatus
and Aspergillus niger in submerged
fermentation system using pectin extracted from mango, orange and pineapple
peels as carbon sources. Nigerian J. Biotechnol. 2014, 28, 26-34.
115. Tai
Y.; Shen J.; Luo Y.; Qu H.; Gong X. Research progress on the ethanol
precipitation process of traditional medicine. Chin Med. 2020, 15, 84.
https://doi.org/10.1186/s13020-020-00366-2
116. Widyarani
A.; Bowden N.A.; Kolfschoten R.C.; Sanders J.P.M.; Bruins M. Fractional
precipitation of amino acids from agro-industrial residues using ethanol. Ind. Eng. Chem Res. 2016, 55(27), 7462-7472. https://doi.org/10.1021/acs.iecr.6b00054.s001
117. Azzaz H.H.; Murad
H.A.; Kholif A.M.; Morsy T.A.; Mansour A.M.; El-Sayed H.M. Pectinase
production, optimization and its application in banana fiber degradation. Egypt J. Nutr. Feed (EJNF), 2013, 16(2), 117-125.
118. Al-Najada A.R.; Al-Hindi R.R.; Mohamed
S.A. Characterization of polygalacturonases from fruit spoilage Fusarium
oxysporum and Aspergillus tubingensis. Afr. J. Biotechnol. 2012, 11,
8527-8536. https://doi.org/10.5897/ajb12.355
119. Chen A.Y.; Adamek R.N.; Dick B.L.;
Credille C.V.; Morisson C.N.; Cohen S.M.
Targeting metalloenzymes for therapeutic intervention. Chem Rev. 2019, 119(2), 1323-1455. https://doi.org/10.1021/acs.chemrev.8b00201.
120. Mao Y.; Robinson J.P.; Binner E.R.
Current status of microwave-assisted extraction of pectin. Chem. Eng. J. 473,
2023, 145261. https://doi.org/10.1016/j.cej.2023.145261
121. Fu M.; Li D.; Sun X.; Fei C.; Zhang D.;
Tuo X.; Gao S.; Han X.; Wang J.; Li Y. Optimization and characterization of
pectin extracted from hawthorn by deep eutectic solvent. Int. J. Biol. Macromol.,
2023, 128688, ISSN 0141-8130, https://doi.org/10.2139/ssrn.4494586.
122. Benvenutti L.; Zielinski A.A.F.;
Ferreira S.R.S. Subcritical water extraction (SWE) modified by deep eutectic
solvent (DES) for pectin recovery from a Brazilian berry by-product. J. Supercri.
Fluids, 189, 2022, 105729. ISSN 0896-8446. https://doi.org/10.1016/j.supflu.2022.105729
123. Chandel V.; Biswas D.; Roy S.; Vaidya D.; Verma A.; Gupta A. Current advancements in pectin: extraction, properties and multifunctional applications. Foods, 2022, 11, 2683. .https://doi.org/10.3390/foods11172683.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
Pectin is known as a polymer of galaturonic acid.
Pectins are commonly found in plants due to the presence of cell walls; with
the pectins categorised into different classes, owing to the differences of
their solubility in water, percent methyl group and carboxyl group
esterification of the galacturonate units. Pectinases (pectin enzymes) are
biocatalysts that split pectin into simpler forms. Over the years, the
production of this enzyme has been discovered from inexpensive agro wastes and
environmental microorganisms which have been evaluated for their specific
capacities to effectively generate this enzyme. The production of pectinase via fermentation is made possible using
the substrate, pectin, an inducer. Fermentation is the primary mode for pectinase production; as
microorganisms employed in the fermentation process act by degrading the more
complex substrate into simpler forms with the production of energy. Solid-state
and submerged fermentations are the main fermentation methods employed in the
industrial production of pectinases. The existence of the different
types of pectin is indicative of the corresponding pectinases produced, and the
justification for the mode of action during catalysis. Several factors have
been implicated in pectinase production, such as the type of fermentation
method used (solid-state fermentation or submerged fermentation), others are;
the substrate type employed in fermentation, pH, temperature, duration of
fermentation, substrate composition, type of metal ion, extraction solvent
used, and the type of precipitation method used for concentrating the enzyme.
This review gives an overview of pectin and processes leading to the production
of the enzyme and simplifies some major modes of action involved in the
application of these enzymes and some relevant factors for optimum production
and application of the enzyme. The
review also shows advances in the extraction of pectin. Some challenges and next steps for future research
have also been pointed out.
Abstract Keywords
Pectin, pectinase, agro-wastes, fermentation, microorganisms.This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).



