Research Article
Adekunle Orimisan Ojatula
Adekunle Orimisan Ojatula
Corresponding
Author
PhytoMedicine, PhytoPharmacology and Drug Discovery Division, Department of Biological Sciences, School of Science,
Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State,
Nigeria
And
PhytoMedicine, PhytoPharmacology and Drug Discovery Research Group, Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State, Nigeria.
E-mail:
kunletula@yahoo.com, Tel: +2347054619103
Goddidit Esiro Enoyoze
Goddidit Esiro Enoyoze
Department of Biological Sciences, Plant Biology and Biotechnology Unit, Edo State, University, Uzairue, Nigeria
And
PhytoMedicine, PhytoPharmacology and Drug
Discovery Research Group, Olusegun Agagu University of
Science and Technology, Okitipupa, Ondo State, Nigeria.
Abstract
In ethnomedicine, different parts of Psidium guajava have been used as herbal remedies for the treatment and management of various ailments. This study was carried out to determine the dose and duration dependent effects of the administration of aqueous root extract of Psidium guajava on the sexual behaviour, serum concentrations of some reproductive hormones and testes of the experimental rats. Wistar albino rats of both sexes were obtained for the study, where forty (40) male albino rats were randomly divided into four groups of ten rats each. Rats in group 1 (control) were administered 1 mL/kg body weight distilled water, while those in groups 2, 3, and 4 were given 100, 200, and 400 mg/kg body weight, respectively, of aqueous extract of Psidium guajava root in the same volume. Female albino rats were made receptive by hormonal treatment. Oral administration of Psidium guajava root extract at the doses and duration evaluated resulted in increased mount, intromission, and ejaculation frequencies (p < 0.05). The latencies of mount and intromission were significantly decreased and ejaculation latency was prolonged. Administration of the extract also reduced the post-ejaculatory interval significantly. The extract increased the computed male sexual behavioural parameters as well as serum hormone concentrations, compared with the distilled water control. Photomicrograph of the testes revealed focal spermatogenic proliferation in the area biopsied in the treated groups (with low doses). The administration of aqueous root extract of Psidium guajava to male Wistar rats at increasing doses and time duration may enhance their fertility and calls for caution in the prolonged use of the plant in folk medicine.
Abstract Keywords
Aphrodisiac; ethnomedicine; Psidium guajava; complementary therapies; medicine, hormone, testes
1. Introduction
From time immemorial medicinal plants have been used to manage an array of diseases/ailments. Nearly 80% of the population of the world still relies on local medicines and traditional treatments mainly from plant extracts [1, 2]. It is noteworthy that herbal medicine is becoming very popular in developing countries [3]. Today, Nigerian traditional medicines are administered to treat a myriad of health problems including mental disorders, insomnia, broken bones and infertility as well as other reproductive health challenges [4].
It has been acclaimed that the use of aphrodisiacs with a healthy lifestyle can achieve a better sexual life [5]. Sexual relationships are an inevitable part of life. It is among the most important social and biological relationships in human life and sexual health is an important component of an individual’s quality of life and well-being [6]. One of the main aims of marriage is procreation (reproduction) to ensure the continuity of an individual’s lineage and, more importantly, for the sexual fulfilment of both partners. For life to continue, an organism must reproduce itself before it dies [7]. All living organisms strive to achieve this through the process of reproduction, which is the vital process that enables a species to represent itself in the following generation in the form of its offspring.
In humans, reproduction is initiated by the mating of a male with a female in sexual intercourse which facilitates the coming together of sperm and egg for fertilization [8]. In order to have a normal sexual intercourse and sexual fulfilment in males, the male sexual organs and factors relating to erection must function normally. The recurrent or repeated inability of the male to perform a satisfactory sexual function or any disorder that interferes with his full sexual response cycle is termed male sexual dysfunction [9]. Male sexual dysfunction is an important contributor of male infertility with about 30-50% of infertility cases attributed to problems with males alone [10] and there have been reports on the exclusive use of herbal remedies to be one of the most vital approaches accessible to man for the treatment and management of this menace [11-14]. Although, the acclaimed herbal remedies used in the treatment and management of sexual dysfunctions in humans are useful therapeutically, but the prolonged usage of these herbs without proper evaluation has brought about a number of health challenges affecting many couples all over the world in their quest seeking the fruit of the womb [15].
Psidium guajava (common name, guava) L. is an evergreen shrub like tree which reaches a height of 6 to 100 feet. The plant has a wide spreading network of branches. Mostly its branches are curved which display opposite leaves with small petioles of about 3 to 16 cm. The leaves are wide and clear green in color and have clear and prominent veins [16]. It is a tropical tree grown for its fruit. It belongs to the phylum Magnoliophyta, class Magnoliopsida and Myrtaceae family [17]. It has about 133 genera and more than 3,800 species. Psidium guajava and its all parts have an old history of medicinal value [18]. The Guava plant grows widely in the tropic areas because it is a plant that can be grown on a wide range of soils [19]. Roots of Psidium guajava have been considered as erectogenic and spermatogenic agents by the people of Ilaje areas of Ondo State, Nigeria for a long time [20]; but to the best of our knowledge, this is the first time in literature, reporting the erectogenic and spermatogenic evaluation effect of Psidium guajava root. The folkloric medicine of the indigenous people of Ilaje area of Ondo State, Nigeria claimed the Psidium guajava root to be more efficacious and potent in enhancing reproductive activity in males than Sildenafil citrate; and as well, recommends that Psidium guajava could be employed as sources of natural antioxidant boosters for the treatment of free radical implicated in reproductive-oxidative stress disorders [20].
As the use of different parts of Psidium guajava as herbal treatment increases indigenously, it is necessary to evaluate the effects of the aqueous extract of the plant on some male sexual behaviour parameters, reproductive hormones and their testes, using experimental animals to justify its use in human at a dose and under given circumstances, so as to ascertain its possible dose and duration dependent effects.
2. Materials and methods
2.1 Plant material
Fresh roots of Psidium guajava were obtained from Eureka Herbal Garden, IgboEgunrin Community, Ilaje Local Government Area, Ondo State, Nigeria on May 2022. The plant sample was identified and confirmed at the Herbarium of the Department of Biological Science, Botany Programme, Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State, Nigeria with the voucher number OAUSTECHBHx106.
2.2 Preparation of plant material
Fresh roots of Psidium guajava were collected, thoroughly washed and air-dried inside the laboratory until a constant weight was obtained. They were pulverized using an electric blender (RN4S, Mayer, China) and sieved to obtain the powdered form. One thousand two hundred grams (1,200 g) of the powdered form was extracted in 99% absolute aqueous using the Soxhlet apparatus. The extraction was carried out in cycles at a temperature of 50 OC, and each cycle lasted for 48 hours. The extract was evaporated to near dryness and as well, concentrated in a water bath under reduced pressure and low temperature. The slurry from the aqueous extract was later weighed and reconstituted in distilled water to give the required doses used in the study.
2.3 Drugs, assay kits and other reagents
Estradiol benzoate and progesterone were purchased from Sigma-Aldrich from China and USA. The testosterone assay kits for test of reproductive hormones were procured from Monobind Inc., USA, while every other chemical used were of analytical grade.
2.4 Experimental animals
The method reported by Sumanta et al. [21] was employed for the procurement of experimental animals, where sexually matured, healthy, albino rats of Wistar strain (Rattus norvegicus), weighing about 230-300 g (male), and 150-180 g (female) were obtained from the animal holding unit of the Department of Pharmacology and Toxicology, University of Benin, and were used for the experiments. The animals were allowed to undergo acclimatization period of seven (7) days as described by Sumanta et al. [21] and were housed in a ventilated wooden cage. They were kept at room temperature 28 – 30 OC under natural light and dark cycle with free access to pelleted feed and tap water. Good hygiene was maintained by constant cleaning and removal of faeces from the cage on a daily basis.
2.4.1 Animal grouping and administration of the extract
Wistar rats of both sexes were obtained and used for the study. A total of 40 male rats, 3 months old (weighing 230–300 g) were selected and randomly divided into four groups and ear tags and colour codes were given to identify each animal. The control group received 1 mL/kg of distilled water for 21 days. The extract was prepared and suspended in 1 mL/kg distilled water and administered orally to the animals with the help of an intragastric catheter at desired dose. The three test groups were administered orally with the aqueous root extract of Psidium guajava on a daily dosage of 100, 200 and 400 mg/kg body weight respectively for 21 days.
Two days (48 hours) prior to the commencement of the experiment, female rats 2.5 months old (weighing 150–180 g) were selected and each of them was administered with estradiol benzoate (10 µg/kg). Four hours prior to the exposure to males, each female rat was also given subcutaneous injection of progesterone (0.5 mg/kg) to ensure that the female rats were in oestrous, this being the time when they were most receptive to fertilization [21] Half hour after the dose administration on day 21 (after seven doses, once daily), day 21 (after fourteen doses, once daily) and day 21 (after twenty one doses, once daily), three male rats from each of the group were individually placed in separate cages and were monitored on experimental periods of days 7, 14 and 21 for sexual/mating behaviour.
2.5 Determination of the aqueous extract of Psidium guajava root on male rats
sexual/mating behaviour
The male sexual behaviour test was carried out by the methods of Dewsburry and Davis [22] and that of Agmo [23] modified by Amin et al. [24] and Yakubu et al. [21].
The receptivity of the females was confirmed before the test by exposing them to the male rats that were not used for the experiments. Receptive females (observed when females firmly raised their hind limb quarters and tails to accept male sexual advances) were selected for the study. The experiment was carried out 2 to 3 hours for diurnal doses on days 7, 14 and 21 on every onset of the dark in a quiet room (under red light), as this is the time when the Wistar rats are most active [2].
Two independent observers, blind to the conditions (test versus control) manually scored by monitoring the behaviour of the male rats. The male rats in separate cages were allowed 10 minutes adaptation period with the receptive females (1 female to 1 male). The occurrence of events and phases of mating after the video recording were analysed and the frequencies and phases were determined. The parameters of male sexual behaviour that were monitored for 15 minutes observation period after pairing includes:
2.5.1 Mount frequency (MF)
The number of times the males assumed copulatory position but failed to achieve intromission – characterized by lifting of the male’s fore body over the hindquarter of the female and clasping her flanks with his forepaw in attempting intromission.
2.5.2 Intromission frequency (IF)
The number of vaginal penetration made by the male during intromission.
2.5.3 Ejaculation frequency (EF)
The number of times there was the expulsion of semen by the males after vaginal penetration were characterized by the rhythmic contraction of the posterior abdomen. The female rats were also observed for the presence of vaginal plugs. In addition, other standard parameters of sexual behaviour obtained through manual data acquisition using stopwatch was included.
2.5.4 Mount latency (ML)
The time from the introduction of the female until the first mount made by the male.
2.5.5 Intromission latency (IL)
The time from the introduction of the female until the first intromission by the male that is usually characterized by pelvic thrusting and springing dismount.
2.5.6 Ejaculation latency (EL)
The time from the first intromission until ejaculation is usually characterized by longer, deeper pelvic thrusting and slow dismounting, followed by a period of reduced activity.
2.5.7 Post ejaculatory interval (PEI)
The time interval from ejaculation to intromission of the next series. Some additional male sexual behaviour parameters computed following the procedures outlined by Yakubu et al. [21] include:
2.6 Test for libido
The level of sexual desire of the male rats was assessed by the protocol outlined in [25]. The libido test was carried out using the mounting and intromission frequencies as well their ejaculation at the mating behavioural test during the 7th, 14th and 21st day.
2.7 Acute Toxicity Study
Forty (40) male rats were utilized in this study and the extract was given as stated in the mating behavioural study earlier. The animals were completely randomized into four groups of ten rats each. In all the groups the animals were monitored for 2 h for any behavioural changes such as hyperactivity, sedation, salivation, diarrhea, accelerated breathing, tail posture and convulsions after administering the extract (at the respective doses- 100 mg/kg, 200 mg/kg and 400 mg/kg), and distilled water to the corresponding groups. The mortality or lethality was counted after 24 h and the Lethal Dose (LD50) was determined. All animals were further observed for up to 21 days for any delayed mortality.
2.8 Sacrifice of animals
At the end of each experimental period (i.e. at day 7, day 14 and day 21), a transverse incision was made through the ventral wall of the abdomen of each rat under slight cervical dislocation. The testes were excised and fixed in Bouin’s fluid in readiness for routine histological procedure. Blood samples were also obtained from the descending abdominal aorta and homogenized in a plain bottle for hormonal assay estimation.
2.9 Biochemical assay
2.9.1 Determination of serum testosterone concentrations
The serum testosterone concentrations of the rats were determined on days 7, 14 and 21 of treatment, ditto luteinizing hormone concentrations and follicle stimulating hormone concentrations. Serum Testosterone was assayed from blood obtained from descending abdominal aorta. The samples were assayed in batches from a standardized curve using the enzyme linked immunosorbent assay (ELISA) method [26]. The microwell kits used were from Syntro Bioresearch Incorporated, California USA.
2.9.2 Determination of luteinizing hormone concentrations
The BioCheck LH ELISA is based on the principle of a solid phase enzyme-linked immunosorbent assay [26]. The assay system utilizes sheep polyclonal anti-LH for solid phase (microtiter wells) immobilization, and a mouse monoclonal anti-LH in the antibody enzyme (horseradish peroxidase) conjugate solution.
2.9.3 Determination of follicle stimulating hormone concentrations
This assay was carried out using double antibody radio immuno-assay. A rat recombinant FSH (I1100) from Amersham, UK was used. The sensitivity of the assay was 0.9 ng/ml [26]. The concentrations of the studied parameters in the serum were evaluated using the method described by Tietz [26] in accordance with the manufacturer’s instruction manual of the used microwell assay kits. Other parameters assayed for in the testicular supernatant were total testicular protein concentration, and total testicular glycogen concentration described by Gornall et al. [27], as well as total testicular cholesterol concentration based on the reaction outlined by Fredrickson et al. [28].
2.10 Histological procedure
The tissues were fixed in Bouin’s fluid for less than 24h. The tissues were then processed via paraffin wax embed method of Drury and Wallington [29] and Scheehan and Brapchak [30]. Staining of the tissues was done using haematoxylin and eosin staining (H & E) dyes method.
2.11 Photomicrography
The sections of the testes of experimental rats/animals were examined under a Leica DM750 research microscope with a digital camera Leica ICC50 attached. Digital photomicrographs of the testes sections were taken at various magnifications.
2.12 Statistical Analysis
The data generated were analyzed using descriptive and inferential statistics. Data were presented as Mean ± Standard Error of Means (S.E.M). The significance difference of means was determined using one-way analysis of variance (ANOVA) at 95% confidence interval. Least Square Difference (LSD) and Duncan multiple range tests were carried out for all groups. All statistical analysis was carried out using Statistical Package for Social Sciences (SPSS) (version 20) manufactured by International Business Machine Corporation (IBM) in Armonk, New York.
3. Results
3.1 Effect of the aqueous extract of Psidium guajava root on sexual/mating behaviour
The administration of the aqueous extract of P. guajava root to male rats on day 7 at a dosage of 100, 200 and 400 mg/kg body weight did manifest a noticeable effect on the sexual behaviour parameters investigated. The treated group of rats exhibited increased (P > 0.05) mount frequency (MF), intromission frequency (IF), ejaculation frequency (EF) and ejaculation latency (EL) as well as decreased (P > 0.05) mount latency (ML), intromission latency (IL) and post-ejaculatory interval (PEI) when compared to the control (Table 1).
Table 1. Effect of Psidium guajava aqueous root extract on male rats mating behaviours monitored on day 7
Groups | MF (time/sec) | IF (time/sec) | EF (time/sec) | ML (sec-1) | IL (sec-1) | EL (sec-1) | PEI (sec-1) |
Control | 0.65±0.31 | 0.43±0.31 | 0.32±0.13 | 3.00a±2.63 | 5.67a±5.67 | 0.67±0.61 | 7.31a±3.31 |
P. guajava (100mg/kg) | 2.65±0.31 | 2.01±1.16 | 1.01±0.78 | 1.65c±0.65 | 2.01b±1.16 | 2.35±1.16 | 2.33b±1.45 |
P. guajava (200mg/kg) | 4.31±0.88 | 3.00±0.58 | 1.65±0.31 | 1.00c±0.01 | 1.65b±0.65 | 2.65±1.20 | 1.00c±0.58 |
P. guajava (400mg/kg) | 0.65±0.31 | 0.31±0.33 | 0.33±0.31 | 3.00b±1.75 | 7.33a±7.31 | 0.67±1.45 | 7.33a±6.84 |
n = 4, P < 0.05 – Significant, Different letters in superscript across the columns are significant from others, Where: MF= Mount Frequency; IF= Intromission Frequency; EF=Ejaculation Frequency; ML= Mount Latency; IL= Intromission Latency; EL= Ejaculation Latency; and PEI= Post - Ejaculatory Interval. | |||||||
Following the administration of the plant extract on day 14, the male rats, upon introduction, responded with immediate advances towards the female and displayed precopulatory behaviour such as chasing, and anogenital sniffing which eventually culminated into the mounting. The extract at a dosage of 100 and 200 mg/kg body weight was able to increase the mount frequency, intromission frequency, ejaculation frequency and ejaculation latency, and had significant decrease (P < 0.05) in mount latency, intromission latency and post-ejaculatory interval when compared to the control. The highest extract dosed group (400 mg/kg body weight) increased (P > 0.05) the mounting frequency, intromission frequency, ejaculation frequency and ejaculation latency at day 14, and caused decreased effect in the mounting latency, intromission latency and post-ejaculatory interval when compared with other dosed groups (Table 2).
Table 2. Effect of Psidium guajava aqueous root extract on male rats mating behaviours monitored on day 14
Groups | MF (time/sec) | IF (time/sec) | EF (time/sec) | ML (sec-1) | IL (sec-1) | EL (sec-1) | PEI (sec-1) |
Control | 0.67±0.33 | 0.43±0.33 | 0.32±0.13 | 5.00a±2.65 | 7.67a±5.67 | 0.67±0.63 | 7.33a±3.33 |
P. guajava (100mg/kg) | 2.67±0.33 | 2.00±1.16 | 1.00±0.58 | 1.67c±0.67 | 2.00b±1.16 | 2.33±1.16 | 2.33b±1.45 |
P. guajava (200mg/kg) | 4.33±0.88 | 3.00±0.58 | 1.67±0.33 | 1.00c±0.00 | 1.67b±0.67 | 2.67±1.20 | 1.00c±0.58 |
P. guajava (400mg/kg) | 0.67±0.33 | 0.33±0.31 | 0.33±0.31 | 3.00b±1.73 | 7.33a±7.31 | 0.67±1.45 | 7.33a±6.84 |
n = 4, P < 0.05 – Significant, Different letters in superscript across the columns are significant from others. Where: MF= Mount Frequency; IF= Intromission Frequency; EF=Ejaculation Frequency; ML= Mount Latency; IL= Intromission Latency; EL= Ejaculation Latency; and PEI= Post - Ejaculatory Interval. | |||||||
At day 21, the various doses evaluated (100, 200 and 400 mg/kg body weight of the extract) showed increased (P < 0.05) effect on the mount frequency, intromission frequency, ejaculation frequency and ejaculation latency parameters, while mount latency, intromission latency and post-ejaculatory interval decreased (P < 0.05) significantly compared to the control (Table 3).
Table 3. Effect of Psidium guajava aqueous root extract on male rats mating behaviours monitored on day 21
Groups | MF (time/sec) | IF (time/sec) | EF (time/sec) | ML (sec-1) | IL (sec-1) | EL (sec-1) | PEI (sec-1) |
Control | 0.67b±0.33 | 0.33±0.13 | 0.33±0.13 | 5.00a±2.65 | 7.67a±5.63 | 0.67±0.33 | 7.33a±5.31 |
P. guajava (100mg/kg) | 2.67a±0.33 | 2.00±1.15 | 1.00±0.58 | 1.67b±0.67 | 2.00b±1.15 | 2.00±1.15 | 2.33b±1.45 |
P. guajava (200mg/kg) | 4.33a±0.88 | 3.00±0.58 | 1.67±0.33 | 1.00b±0.00 | 1.67b±0.67 | 2.67±1.20 | 1.33c±0.33 |
P. guajava (400mg/kg) | 3.67a±0.33 | 2.67±0.33 | 1.53±0.33 | 1.17b±1.73 | 2.33b±0.88 | 2.27±0.67 | 3.00b±1.53 |
n = 4, P < 0.05 – Significant, Different letters in superscript across the columns are significant from others. Where: MF= Mount Frequency; IF= Intromission Frequency; EF=Ejaculation Frequency; ML= Mount Latency; IL= Intromission Latency; EL= Ejaculation Latency; and PEI= Post - Ejaculatory Interval. | |||||||
The computed male rats sexual behaviour parameters which include percentage (%) index of libido, % mounted, % intromitted, % ejaculated, intromission ratio and % copulatory efficiency were significantly (P < 0.05) higher in the extract treated rats except for the intercopulatory efficiency, which was reduced when compared to the control group (Table 4).
Table 4. Effect of Psidium guajava aqueous root extract on computed male rat sexual behaviour parameters
Groups | Index of Libido (%) | Mounted (%) | Intromitted (%) | Intromission Ratio | Ejaculated (%) | Copulatory Efficiency (%) | Intercopulatory Interval (Efficiency) (sec.) |
Control | 17.67d | 35.33c* | 17.67d | 0.33 | 18.67c* | 50c* | 192c* |
P. guajava (100mg/kg) | 50b | 66.66b | 50b | 0.44 | 33b | 80a* | 132a* |
P. guajava (200mg/kg) | 100a | 100a | 100a | 0.64 | 83a | 100a | 112a |
P. guajava (400mg/kg) | 64.66b | 64.66b | 64.66b | 0.46 | 50b | 85.71a* | 120a* |
n = 4, *P < 0.05 – Significant, Different letters in superscript across the columns are significant from each other | |||||||
3.2 Effect of Psidium guajava aqueous root extract on libido
The results obtained in the test for libido showed that the root extract of Psidium guajava at the doses of 100, 200 and 400 mg/kg, increased the Mounting Frequency (MF) (P<0.05) when compared to the control group. Intromission was observed in extract treated groups of rats, while it was absent in control, however, ejaculation was noted across the dose levels of extract treated groups of animals used for the test of libido (Table 5).
Table 5. Effect of Psidium guajava aqueous root extract on mount frequency (test for libido) in male rats
Groups | Mount frequency | Intromission frequency | Ejaculation |
Control | 0.43±0.31 | 0.00±0.00 | Absent |
100 mg/kg | 1.33±0.13 | 0.67±0.33 | Present |
200 mg/kg | 2.33±0.88 | 1.67±0.67 | Present |
400 mg/kg | 1.67±0.17 | 1.27±0.33 | Present |
3.3 Acute toxicological study
Results from the acute toxicological studies revealed that the aqueous extract of Psidium guajava root was safe up to the highest dose of 400 mg/kg. No toxic symptoms or adverse behavioural changes were observed as zero mortality was recorded during the period of the study.
3.4 Effect of aqueous extract of Psidium guajava root on testes-body weight
The testes-body weights were 1.32%, 1.38% and 1.31% at the various doses of 100, 200, and 400 mg/kg body weight, respectively while the control value was 1.10%. The administration of aqueous extract of Psidium guajava root increased significantly (P < 0.05) the testes-body weight ratio across all treated groups compared with the control.
3.5 Effect of aqueous extract of Psidium guajava root on reproductive hormonal levels
3.5.1 Effect of the aqueous extract of Psidium guajava root on serum testosterone concentration
The serum testosterone levels were (1.70±0.14), (2.90±0.07) and (1.40±0.14) ng/mL at the various doses of 100, 200, and 400 mg/kg body weight of extract administered, respectively when compared with the control value of (1.00±0.65) ng/mL. The highest increase observed was detected in the animals that received extract concentration of 200 mg/kg body weight while 400 mg/kg body weight of aqueous extract had the lowest value. The serum testosterone level of the rats treated with aqueous extract of Psidium guajava root was observed to increase significantly (P < 0.05); this increase was not observed to be significant in animals that were administered 400 mg/kg body weight of the extract.
3.5.2 Effect of the aqueous extract of Psidium guajava root on serum luteinizing hormone (LH) concentration
Luteinizing hormone (LH) levels were (3.50±0.21), (2.30±0.14) and (1.90±0.28) mlu/Ml at the various doses administered when compared with the control which was (1.30±0.07) mlu/mL. The highest increase was detected in the animals that received extract concentration of 100 mg/kg body weight which was 3.50 mlu/mL, 200 mg/kg body weight of aqueous extract had 2.30 mlu/mL as the observed value while 400 mg/kg body weight of aqueous extract had the lowest value which was observed to be 1.90 mlu/mL. When compared with the control, the level of the serum LH increased significantly (P < 0.05) but this showed a counter dose-dependent manner.
3.5.3 Effect of the aqueous extract of Psidium guajava root on serum follicle stimulating hormone (FSH) concentration
Follicle stimulating hormone (FSH) levels were increased to (10.60±0.42), (9.20±0.28) and (4.25±0.25) mlu/mL respectively at the various doses administered when compared with the control which was (3.50±0.35) mlu/mL. The highest increase observed for the serum FSH concentration was detected in the animals that received extract concentration of 100 mg/kg body weight which was observed to be 10.6 mlu/mL, 200 mg/kg body weight of aqueous extract had 9.2 mlu/mL while 400 mg/kg body weight of aqueous extract had the lowest value observed to be 4.25 mlu/mL. A significant increase (P < 0.05) was observed in the FSH level in the animals administered with 100 and 200 mg/kg body weight of extract when compared with the control. However, a non-significant increase was noted in animals that given 400 mg/kg body weight of the aqueous extract of Psidium guajava root.
3.6 Effect of the aqueous extract of Psidium guajava root on testicular functions
3.6.1 Effect of aqueous extract of Psidium guajava root on testicular protein
Total protein levels were (78.72±2.63), (66.13±1.51) and (59.77±1.25) mg/mL respectively at the various doses administered when compared with the control which was (51.58±1.82) mg/mL. The highest increase observed was detected in the animals that received extract concentration of 100 mg/kg; 200 mg/kg body weight of aqueous extract had 66.13 mg/mL while 400 mg/kg body weight of aqueous extract had the lowest values observed to be 59.77 mg/mL. It increased significantly (P < 0.05) the testicular protein level when compared with the control in a counter-dose dependent order; this increase was however not significant in the animals treated with 400 mg/kg body weight.
3.6.2 Effect of aqueous extract of Psidium guajava root on testicular glycogen
Testicular glycogen levels were (7.88±0.62), (6.67±0.47) and (3.81±0.57) mg/100 mg glucose at 100, 200 and 400 mg/kg body weight respectively when compared with the control which was (3.13±0.12) mg/100 mg glucose. The highest increase observed was detected in animals that received extract concentration of 100 mg/kg body weight while the 400 mg/kg body weight had the lowest value. There was a significant increase (P < 0.05) across all treated groups except the 400 mg/kg body weight of the aqueous extract of Psidium guajava root.
3.6.3 Effect of aqueous extract of Psidium guajava root on testicular cholesterol
Testicular cholesterol levels were (2.29±0.21), (2.63±0.45) and (1.21±0.15) mmol/L at the various doses administered when compared with the control which was (0.77±0.01) mmol/L. The highest increase observed was detected in the animals that received extract concentration of 200 mg/kg body weight which was 2.63 mmol/L, the 100 mg/kg body weight of aqueous extract had 2.29 mmol/L, while the 400 mg/kg body weight of aqueous extract had the lowest value which was observed to be 1.21 mmol/L. The effect of aqueous extract of Psidium guajava root when compared with the control showed a significant increase in testicular cholesterol level.
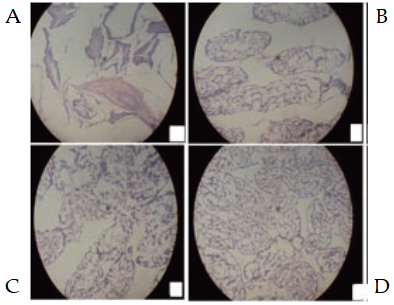
3.7 Evaluation of spermatogenic proliferation
Figure 1 displayed the photomicrographs of the testes of rats administered with aqueous extract of Psidium guajava root. The testis section of the control group animals (Figure 1A) showed normal histological texture. The diameter of seminiferous tubules varied within a range. The tubules having maximum diameter, were not abundant but textured within range. The cuboidal germinal epithelium exhibited normal shape and size. Sertoli cells had many cytoplasmic processes which were normal in size. Spermatozoa were embedded in the sertoli cells and showed normal cytoplasmic granulation. Leydigs cells had normal nuclear size. Luminal part of the tubules were normal in number with bundles of spermatozoa. Spermatozoa with long tail with small distinct head were visible microscopically (Figure 1A). The extract treated group of rats showed pronounced similar effects in terms of testis weight and non-histological alterations when compared with the control; as evident in Figure 1B and Figure 1C. The administration of P. guajava root extract at all dose levels, revealed sperm cells in normal sequential maturation coupled with normal cytoplasmic granulation, and seminiferous tubules with spermatogenic series and interstitial spaces. Also, increment in the volume of cells and nucleus was strongly suggestive of steroid synthesis under the direct or indirect influence of the extract. Almost all tubules were overcrowded with sperm bundles (Figure 1B-D), when viewed microscopically. Although, the aforementioned spermatogenic potentials were found prominent in the group of rats that received 200 mg/kg body weight of the extract, followed by the 100 mg/kg, while the highest dose of 400 mg/kg had fairly distorted tissue with sequential maturation of sperm cells (Figure 1D).
Figure 1. Micrographs of testes of male Wistar rats administered with aqueous extract of Psidium guajava root. A: Control (1 mL/kg of distilled water); B: 100 mg/kg body weight; C: 200 mg/kg body weight; and D: 400 mg/kg body weight.
4. Discussion
4.1 Mating behavioural study
This study focused on the dose and duration effects of aqueous root extract of Psidium guajava on some sexual behaviour parameters, reproductive hormones and histology of the testes of the experimental rats. Using animal models for the initial screening to determine the aphrodisiac potential of a test drug is an accepted model. The employed model used in this research study is simple and quick [31], and can also be said to be used to evaluate the aphrodisiac and stimulating activity on penile erection against erectile dysfunction [32]. Sexual behavioural parameters such as mount and intromission frequencies are indices of sexual vigour, libido and potency [33, 34]. The mating behavioural test revealed that the aqueous extract of P. guajava root increased mount and intromission frequencies when compared with the control group (Tables 1, 2 and 3). It also decreased the mount and intromission latencies in the male rats (Tables 2 and 3) and prolonged the ejaculatory latencies on days 7, 14 and 21. These significant increases in mounting frequencies (MF) and intromission frequencies (IF) with corresponding decreases in mount latency (ML) and intromission latency (IL) are indications that the male rats were aroused. It also reflects enhanced performance, motivation and vigour. These findings agree with earlier reports by Ratnasooriya and Dharmasiri [35]; Yakubu and Afolayan, [36]; Yakubu and Akanji [4]; Gbankoto et al. [37]; Ojatula et al. [38]; Ojatula et al. [39] on the significant changes in ML and IL. Also, the prolonged ejaculatory latency (EL) by the aqueous extract of P. guajava is a strong indication that the sexual function of the male rats was enhanced (prolonged duration of coitus) suggesting an aphrodisiac activity. These findings which is similar to the report by Fouche et al. [40]; Ojatula et al. [39] further support the activity of P. guajava root aqueous extract in enhancing sexual function. However, the highest dose of 400 mg/kg had decreased activity/function on sexual behavioural parameters on day 21 (Table 3), when compared to administered doses of extract on days 7 and 14. This agreed with the findings of Ratnasooriya and Dharmasiri [35]; Yakubu and Afolayan [34]; Ojatula et al. [39] where they observed the same reverse inhibition at the highest dose of 3000 mg/kg of Terminalia catappa seeds and 100 mg/kg of Bulbine natalensis stem in their respective studies. This may be due to sedation as animals showed decreased form of sexual interest.
4.2 Male reproductive hormones
It has been earlier reported that androgens are important modulators of male sexual behaviour including erection and libido. These androgens may act both at the central and peripheral nervous system levels [41, 42]. Furtherance to the action of androgen on central and peripheral nervous system levels, one may exercise the quest seeking the effect of herbal remedies on testicular compartment. Therefore, this present study displays in the experimental rats an overall elevation in the testes-body weight ratio after 21-days of oral administration of the aqueous extract of Psidium guajava root. This significant increase observed in the testes-body weight may be as a result of increased synthesis of testosterone and androgen since testes is an androgen dependent organ [43, 44]. The study also reveals a direct relationship between testosterone level and testes-body weight. This finding corroborates the observation of Morakinyo et al. [45] and Ojatula [46], where they worked on the effect of herbal extract on spermatogenesis of experimental rats. Also, it was noted that the administration of the aqueous extract of Psidium guajava root to experimental animals for 21 days increased significantly the serum testosterone concentration compared with the control. Testosterone secretion is stimulated by LH and its role is to enhance the growth and secretory activity of the testes [47]. The increase in testosterone levels observed in this study corroborates those of Etuk and Muhammad [48] and Ojatula et al. [39], where they worked on the fertility enhancing effects of aqueous stem bark extract of Lophira lanceolata in male Sprague Dawley rats, and aphrodisiac potentials of Pausinystalia yohimbe root in male Wistar rats; however, this result contradicts the result of Morakinyo et al. [49], where there was an observed reduction in the level of testosterone in arsenite-treated rats. Administration of aqueous extract of Psidium guajava root in this study to experimental animals increased significantly the level of serum luteinizing hormone (LH). LH is responsible for stimulating testosterone production in leydig cells which subsequently stimulates spermatogenesis by acting on the sertoli cells and peritubular cells of the seminiferous tubules [47]. The result observed in this current study however refutes those of Yakubu [44], where significant decrease in the level of serum LH level was detected after a 60-days oral administration of a crude alkaloid extract from Chromolaena odorata leaves to male rats in his study on spermatogenic and hormonal indices of male rats. In addition, the follicle stimulating hormone (FSH) level was observed in this study to increase in the experimental animals administered with the extract. FSH facilitates the testosterone passage via sertoli-sertoli junctional complexes by acting on sertoli cells, leading to the androgen-binding protein production [50]. This result is in agreement with the findings of Akdogan et al. [51]. In their report, on the effects of peppermint tea on plasma testosterone, LH and FSH in rats, there was an increase in plasma FSH levels during the study.
4.3 Testicular Functions
Following the 21 days of administration of aqueous extract of Psidium guajava root to experimental animals, there was significant increase in testicular protein level after the administration of aqueous extract of Psidium guajava root to male rats for 21 days. This may be a result of the stimulation of growth proteins and ribonucleic acid synthesis [52, .53]. The increase in testicular total protein observed agrees with the result of Joshi et al. [54], where testicular protein content in rats was observed to increase significantly following their 30-day exposure to chlorpyrifos. Testicular glycogen level was observed to increase significantly in this study after the administration of the extract. Testicular glycogen has been associated with a supply of energy for testicular cells and tissue, and an increase in glycogen level may also enhance the synthesis of protein in spermatogenic cells due to its dependence on glucose for energy supply [44]. This observation corroborates with the findings of the testicular protein level from this current study. The observation in this study however negates the report of Yakubu et al. [55]. The level of testicular cholesterol increased significantly following the 21 days’ administration of the extract. Cholesterol is the main precursor of steroid hormones like testosterone and it is also associated with normal testicular function [56]. The significant increase in testicular cholesterol level observed in this study may imply incremental testicular function in the male rats. This result is in line with those of Yakubu and Afolayan [57], where there was significant increase in testicular cholesterol level of male Wistar rats administered with aqueous extract of Bulbine natalensis stem as a result of enhanced anabolic and androgenic activities of the plant.
4.4 Spermatogenic proliferation
Sequel to the enhancement of testicular functions in extract treated group of rats; spermatogenic proliferation, an enhancement of the complex process of germ cell differentiation which leads to the formation of spermatozoa [46], increased in the testes of the extract treated rats as observed in the area biopsied of rats. Histological findings revealed gradual increased focal spermatogenic proliferation in the area biopsied in the group of rats given low doses (100, and 200 mg/kg) of the extract subject to this study (Figure 1B and C); and this results agree with the findings of Ojatula [46], where he worked at low doses on spermatogenic efficacy of Pausinystalia yohimbe (K. Schum.) Piere ex Beille roots in male rats. While the treated group with high dose (400 mg/kg body weight) of the extract showed cloudy distortion of spermatogenic proliferation (Figure 1D). Thus, administration of aqueous root extract of Psidium guajava at graded doses exceeding 400 mg/kg body weight of extract, over a long time, this may gradually induce arrest in spermatogenesis as observed in this study.
4.5 Toxicological evaluation
All animals in all the groups, showed no significant adverse acute toxicological effect that can be attributed to the acute administration of the aqueous extract of P. guajava root. Also, adverse changes in behaviour were not observed, indicating that physical clinical signs were unremarkable. The intake of food and water were normal, suggesting that the animals had a normal appetite. No mortality was noticed during the entire period of the study. It can therefore be inferred that the lethal dose (LD50) of the extract is greater than 400 mg/kg since up to this dose no death was recorded. This finding agrees with previous report by Gatsing et al. [58] where 0 % mortality was recorded when the aqueous leaf extract of Alchornea cordifolia at 3200 mg/kg and the aqueous leaf extracts of Emilia coccinea at 8000 mg/kg were administered [59]. Equally importantly, the foregoing findings as observed, also agree with the previous current scientific reports by Ojatula [60]; Ojatula and Afolabi [61]; Ojatula and Nwanja [62]; Ojatula and Afolabi [63] where extracts administered experimental animals used to justify human circumstances, showed no mortality throughout the duration of the study; thereby, lending credence to the successive enhancement of scientific knowledge and giving future hope on the formulations, usage and safety of herbal plants for sustainable system of good health and well-being.
5. Conclusions
The administration of aqueous root extract of Psidium guajava to male Wistar rats at different (increasing) doses and time (increasing) duration resulted in increases in aphrodisiac effect, serum concentrations of follicle stimulating hormone, luteinizing (FSH), luteinizing hormone (LH) and Testosterone, as well as proliferation of spermatogenesis in their testes. This may enhance fertility and calls for caution in the prolonged use of high doses of the plant in folk medicine.
Authors’ contributions
Experiment and drafting of the manuscript, O.A.O.; Operations of directing, controlling, interpreting the results and checking of the manuscript, O.A.O.; Supervision and final checking of the manuscript, O.A.O.; E.G.E.
Acknowledgements
Third Eyes Research
Laboratory Okitipupa, Ondo State, Nigeria
Funding
During this study, no financial support was received.
Availability of data and materials
All relevant data are within the paper and its supporting information files.
Conflicts of interest
The authors have declared that no competing interests exist.
References
1. WHO. World Health Organisation. Working Document on Sexual and Reproductive Health; Geneva, 2002.
2. Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T. Male sexual dysfunction and methods used in assessing medicinal plants with aphrodisiac potentials. Pharmacog. Rev. 2007, 1, 49-56.
3. Fullick, A. Biology.; Heinemann Advance Science: Oxford, 1994.
4. Yakubu, M.T.; Akanji, M.A. Effect of aqueous extract of Massularia acuminate stem on sexual behaviour of male Wistar rats. Evid. Bas. Complement. Altern. Med. 2011, 1, 73-81.
5. Ekwere, P.D.; Archibong, E.I.; Bassey, E.E.; Ekabbua, J.E.; Ekanem, E.I.; Feyi-Waboso, P. Infertility among Nigerian couples as seen in Calabar. Por. Harc. Med. J. 2007, 2, 35-40.
6. Akerele, O. Summary of WHO guidelines for the assessment of herbal medicine. Herb. Gram. 1993, 28, 13–19.
7. Sagga, S.; Diverkar, H.M.; Gupta, V.; Sawhney, R.C.; Banerjee, P.K.; Kumar, R. Adaptogenic and safety evaluation of seabackthorn (Hippophae rhamnoides) leaf extract: a dose dependent study. Food Chem. Toxixcol. 2007, 45, 609–17.
8. Obici, S.; Otobone, F.J.; Da Silva-Sela, V.R.; Ishida, K.; Da Silva, J.C.; Makamura, C.V.; et al. Preliminary toxicity study of dichloromethane extract of Kielmeyera coriacae stems in mice and rats. J. Ethnopharmacol. 2008, 115, 131–139.
9. Enwereji, E. Views on tuberculosis among the Igbo of Nigeria. Indigen. Know. Develop. 1999, 7, 4–7.
10. Ganu, G.; Nagore, D.H.; Rangari, M.; Gupta, H. Pharmacology evaluation of Ayurvedic plants in experimental animals. J. Complem. Integrat. Med. 2010, 7, 1553–3840.
11. Ojatula, A.O.; Aworinde, D.O.; Osewole, A.O.; Ogungbemi, A.O. Aphrodisiac potential and phytochemical evaluation of aqueous extract of Microdesmis keayana J. Leonard roots. Coast J. Fac. Sci. 2020, 1(2), 154-163.
12. Ojatula, A.O. Spermatogenic efficacy of Pausinystalia yohimbe (K. Schum.) Piere ex Beille roots in male rats. J. Infert. Reprod. Biol. 2020, 8(2), 9-17.
13. Ojatula, A.O.; Idu, M.; Timothy, O. Aphrodisiac potentials of Pausinystalia yohimbe (K. Schum.) Pierre ex Beille maqueous root extract in male Wistar rats. J. Integ. Nephro. Androl. 2020, 7(2), 47-55.
14. Ojatula, A.O. Developmental botanic: a case study on chromatographic determination of phytocompounds in the roots of Anthocleistal nobilis (G. Don.) as pro-fertility. J. Infert. Reprod. Biol. 2021, 9(1), 7-21.
15. Leke, R. Reproductive Health in Cameroon. World Health Organization (WHO) Collaborating Centre for Research in Human Reproduction; Geneva, 2008.
16. Naseer, S.; Hussain, S.; Naureen-Naeem, N.; Pervaiz, M.; Madiha-Rahman, M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytosci. 2018, 4(32), 1-8
17. Dakappa, S.S.; Adhikari, R.; Timilsina, S.S.; Sajjekhan, S. A review on the medicinal plant Psidium Guajava Linn. (Myrtaceae). J. Drug Deliv. Ther. 2013, 3(2), 162–168.
18. Nwinyi, O.; Chinedu, S.N.; Ajani, O.O. Evaluation of antibacterial activity of Pisidium guajava and Gongronema latifolium. J. Med. Plants Res. 2008, 2(8), 189–192.
19. Jiminez-Escrig, A.; Rincon, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49(11), 5489–5493.
20. Erhabor, J.O.; Idu, M.; Ojatula, A.O. Ethnomedicinal survey of plants used in the treatment of male sexual dysfunction among the Ilaje people of Ondo State, Nigeria. In: Kumar, S. (Ed.) Recent Advances in Ethnobotany; Deep publications: Paschim Vihar, New Delhi, India, 2015.
21. Sumanta, K.G.; Manoj, K.P.; Rohitash, J.; Skekhar, D.; Amit, A.; Mohammed, N.I. Screening of Rho-kinase 2 inhibitory activity of Indian medicinal plants for management of erectile dysfunction. J. Ethnopharmacol. 2012, 144, 483-489.
22. Dewsburry, D.A.; Davis, H.N. Effect of reserprine on the copulatory behaviour of male rats. Physio. Behav. 1970, 5, 1331–1333.
23. Agmo, A. Male rat’s sexual behaviour. Bra. Res. Prot. 1997, 1, 203–209.
24. Amin, K.M.Y.; Khan, M.N.; Rahman, S.Z.; Khan, N.A. Sexual function improving effect of Mucuna pruriens in sexually normal male rats. Fitother. 1996, 67, 53–58.
25. Guay, A.T.; Spark, R.F.; Bensel, S.; Cunningham, G.R.; Goodman, N.F.; Nakin, H.R.; et al. Medical guidelines for clinical practice for the evaluation and treatment of male sexual dysfunction: a couple’s problem. Endocrin. Prac. 2003, 9(1), 78-95.
26. Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; WB Saunders Company: Philadelphia, USA, 1995.
27. Gornall, A.C.; Bardawill, C.J.; David, M.M. Determination of serum protein by means of biuret reaction. J. Biol. Chem. 1949, 177, 751-756.
28. Fredrickson, D.S.; Levy, R.I.; Lees, R.S. Fat transport in lipoproteins-an integrated approach to mechanisms and disorders. N. Engl. J. Med. 1967, 276, 148-156.
29. Drury, R.A.; Wallington, E.A. Cartelon’s Histological Techniques. Oxford University Press: London, 1980.
30. Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Hstotechnology, 2nd ed.; The CV Mosby Company: St Louis, USA, 1980.
31. Ang, H.H.; Sim, M.K. Effects of Eurycoma longifolia sack on penile erection index and homosexual mounting in rats. Pharmacol. Sci. 1997, 3, 117–129.
32. Maeda, N.; Matsuoka, N.; Yamazaki, M.; Arakawa, H.Y.; Ohkubo, I.Y. A screening concept based on a hypothesis led to the development of putative cognitive enhancer that stimulates penile erection. Japan. J. Pharm. 1994, 64, 147–153.
33. Tajuddin, A.S.; Latif, A.; Quasmi, I.A. Effect of 50% aqueous of Syzygium aromaticum (L) Merr. and Perry. (Clove) on sexual behavior of normal male rats. BMC Compl. Altern. Med. 2004, 4, 17–24.
34. Mbongue, F.G.Y.; Kamtchouing, P.; Essame, O.J.L.; Yewah, P.M.; Dimo, T.; Lontsi, D. Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Ind. J. Pharmac. 2005, 37, 30–42.
35. Ratnasooriya, N.D.; Dharmasiri, M.G. Effect of Terminalia catappa seeds on the sexual behaviour and fertility of male rats. Asian J. Androl. 2000, 2, 213–219.
36. Yakubu, M.T.; Afolayan, A.J. Effect of aqueous extract of Bulbine natalensis (Baker) stem on the sexual behavior of male rats. Int. J. Androl. 2008, 32, 629–36.
37. Gbankoto, A.; Anago, E.; Houndjo, P.A.; Adjahouinou, D.C.; Gbaguidi, F. Effect of aqueous and aqueousic extracts of Caesalpinia bonduc root on sexual behaviour of male Wistar rats. Int. J. Multidiscipl. Curr. Res. 2015, 3, 1137–1141.
38. Ojatula, A.O. Methanol Root Extract of Pausinystalia yohimbe, an aphrodisiac influences morphology of sperm cells in male Wistar rats. J. Curr. Biomed. Res. 2022, 2(3), 160-170.
39. Ojatula, A.O. Aphrodisiac potentials of Pausinystalia yohimbe (K. Schum.) Pierre ex Beille maqueous root extract in male Wistar rats. J. Integra. Nephrol. Androl. 2020, 7(2), 47-55.
40. Fouche, G.; Afolayan, A.J.; Wintola, O.A.; Khorombi, T.E.; Senabe, J. Effect of the aqueous extract of the aerial parts of Monsonia angustifolia E. Mey. Ex a. Rich., on the sexual behaviour of male Wistar rats. BMC Complement. Altern. Med. 2015, 15, 343–53.
41. Mills, T.M.; Reilly, C.M.; Lewis, R.W. Androgens and penile erection: a review. J. Androl. 1996, 17, 635–658.
42. Morelli, A.; Filippi, S.; Vignozzi, L.; Mancina, R.; Maggi, M. Physiology of erectile function: an update on intracellular molecules processes. EAU–EBU Update Ser. 2006, 4, 96–108.
43. Klinefelter, G.R.; Hess, R.A. Toxicology of the Male Excurrent Ducts and Accessory Sex Glands. In: Korach, K.S.; Editor. Reproduction and Developmental Toxicology. Marcel Dekker Incorporated: New York, London, 1998.
44. Yakubu, M.T. Effect of a 60-day oral gavage of a crude alkaloid extract from Chromolaena odorata leaves on hormonal and spermatogenic indices of male rats. J. Androl. 2012, 33(6), 1199-1207.
45. Morakinyo, A.O.; Adeniyi, O.S.; Arikawe, A.P. Effects of Zingiber officinale on reproductive functions in the male rat. Afr. J. Biom. Res. 2008, 11, 329-334.
46. Ojatula, A.O.; Nwanja, N. In-vitro studies of the medicinal plant Microdesmis keayana methanol leaves extract phytocomponents by GCMS analysis: A further evidence for its phytomedicinal potentials in enhancement of reproductive functions. Arid-Zone J. Bas. Appl. Res. 2023, 2(1), 192-202.
47. Parandin, R.; Yousof, N.; Ghorbani, R. The enhancing effects of alcoholic extract of Nigella sativa seed on fertility potential, plasma gonadotropins and testosterone in male rats. Iran. J. Reprod. Med. 2012, 10(4), 355-362.
48. Etuk, E.U. Muhammad AA. Fertility enhancing effects of aqueous stembark extract of Lophira lanceolata in male Sprague Dawley rats. Int. J. Plant Physiol. Biochem. 2009, 1(1), 001-004.
49. Morakinyo, A.O.; Achema, P.U.; Adegoke, O.A. Effect of Zingiber officinale (ginger) on sodium arsenite-induced reproductive toxicity in male rats. Afr. J. Biom. Res. 2010, 13, 39-45.
50. D’Cruz, S.C.; Vaithinathan, S.; Jubendradass, R.; Mathur, P.P. Effects of plants and plants products on the testis. Asian J. Androl. 2010, 12, 466-479.
51. Akdogan, M.; Ozguner, M.; Kocak, A.; Oncu, M.; Cicek, E. Effects of peppermint teas on plasma testosterone, follicle stimulating hormone, and luteinizing hormone levels and testicular tissue in rats. Urol. 2004, 64, 394-398.
52. Puga, F.R.; Rudrigues, F.R.; Lark, M.A. Effect of dimethoate and propoxur on the metabolism of IB-RS2 cells and their susceptibility to foot and mouth disease. Sau Paulo, Brazil: Arquivos do Institu Biologico. 1974, 141-144.
53. Dutta, A.L.; Sahu, C.R. Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Spring. Plus. 2013, 2, 54-61.
54. Joshi, S.C.; Mathur, R.; Gulati, N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rat. Toxicol. Ind. Health. 2007, 23, 439-444.
55. Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T. Evaluation of antiandrogenic potential of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Androl. 2007, 39, 235-243.
56. Nurudeen, Q.O.; Ajiboye, T.O. Aqueous root extract of Lecaniodiscus cupanioides restores the alterations in testicular parameters of sexually impaired male rats. Asian Pac. J. Reprod. 2012, 1(2), 120-124.
57. Yakubu, M.T.; Afolayan, A.J. Anabolic and androgenic activities of Bulbine natalensis stem in male Wistar rats. Pharm. Biol. 2010, 48(5), 568-576.
58. Gatsing, D.; Nkeugouapi, C.F.N.; Nkah, B.F.N.; Kuiate, J.R.; Tchouanguep, F.M. Antibacterial activity, bioavailability and acute toxicity evaluation of the leaf extract of Alchornea cordifolia (Euphorbiaceae). Int. J. Pharm. 2010, 6, 173–82.
59. Idu, M.; Erhabor, J.O.; Ovuakporie, U. Ethnomedicinal plants used by the Idoma people– Benue State, Nigeria. Amer. J. Ethnomed. 2014, 1(1), 072–088.
60. Ojatula, A.O. Preliminary assessment of acute and 14-days repeated dose oral toxicity of Pausinystalia yohimbe (K. Schum.) Pierre ex Beille methanol root extract on experimental rats. PhytoChem. BioSub. J. 2020, 14(3), 191-202.
61. Ojatula, A.O.; Afolabi, F.O. Aphrodisiac plant Pausinystalia yohimbe mnduces myocardiac histological normalcy in Wistar rats. J. Appl. Sci. Environ. Manage. 2022, 26(8), 1385-1389.
62. Ojatula, A.O.; Nwanja, N. Effects of a conventional aphrodisiac: a twenty eight day repeated administration of Sildenafil citrate graded doses on experimental rats. Dutse J. Pure Appl. Sci. (DUJOPAS), 2022, 8(3b), 115-123.
63. Ojatula, A.O.; Afolabi, F.O. Preliminary phytochemistry, minerals concentration determination and acute oral toxicity study of Nigerian’s Azadirachta indica (A. Juss). Bokkos J. Appl. Sci. Rep. 2023, 2(2), 71-83.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
In ethnomedicine, different parts of Psidium guajava have been used as herbal remedies for the treatment and management of various ailments. This study was carried out to determine the dose and duration dependent effects of the administration of aqueous root extract of Psidium guajava on the sexual behaviour, serum concentrations of some reproductive hormones and testes of the experimental rats. Wistar albino rats of both sexes were obtained for the study, where forty (40) male albino rats were randomly divided into four groups of ten rats each. Rats in group 1 (control) were administered 1 mL/kg body weight distilled water, while those in groups 2, 3, and 4 were given 100, 200, and 400 mg/kg body weight, respectively, of aqueous extract of Psidium guajava root in the same volume. Female albino rats were made receptive by hormonal treatment. Oral administration of Psidium guajava root extract at the doses and duration evaluated resulted in increased mount, intromission, and ejaculation frequencies (p < 0.05). The latencies of mount and intromission were significantly decreased and ejaculation latency was prolonged. Administration of the extract also reduced the post-ejaculatory interval significantly. The extract increased the computed male sexual behavioural parameters as well as serum hormone concentrations, compared with the distilled water control. Photomicrograph of the testes revealed focal spermatogenic proliferation in the area biopsied in the treated groups (with low doses). The administration of aqueous root extract of Psidium guajava to male Wistar rats at increasing doses and time duration may enhance their fertility and calls for caution in the prolonged use of the plant in folk medicine.
Abstract Keywords
Aphrodisiac; ethnomedicine; Psidium guajava; complementary therapies; medicine, hormone, testes
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


