Research Article
Stephen Oluwayinka Olayemi
Stephen Oluwayinka Olayemi
Faculty of Pharmacy, Obafemi
Awolowo University,Ile-Ife, Nigeria
Freedom Amarachi Nkwocha
Freedom Amarachi Nkwocha
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Etu Esther Ifeyinwa
Etu Esther Ifeyinwa
Department
of Anatomic Pathology, Alex Ekweme Federal University, Ndufu-Alike, Nigeria
Chikamso Favour Agbo
Chikamso Favour Agbo
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Chukwu Chigozie David
Chukwu Chigozie David
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Chukwu Chidimma Mebrim
Chukwu Chidimma Mebrim
Department
of Medical Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Sunday Moses Akuaden
Sunday Moses Akuaden
Department
of medical laboratory science, Bamenda University of science and technology, Cameroon.
Wanger Jaclyn Tachia
Wanger Jaclyn Tachia
Department
of Public Health and
Healthcare, I.M Sechenov First Moscow State
Medical University, Moscow, Russia.
Reuben Chukwuma Odo
Reuben Chukwuma Odo
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Chiagozie Chinyere Umeano
Chiagozie Chinyere Umeano
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Kingsley Uchechukwu Eke
Kingsley Uchechukwu Eke
Department of Medical Laboratory science, Abia State University Uturu, Abia State, Nigeria
Ozeh Chidimma Deborah
Ozeh Chidimma Deborah
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Nweke Sixtus Chinecherem
Nweke Sixtus Chinecherem
Department of Medical
Laboratory Sciences, University of Nigeria, Nsukka, Nigeria.
Justice Chibuikem Olelewe
Justice Chibuikem Olelewe
Department of Medical Laboratory Science, University
of Jos, Plateau State, Nigeria.
Uyi Eunice Osayamwen
Uyi Eunice Osayamwen
Department of Animal and Environmental Biology, Faculty of Life sciences, University: University
of Benin, Edo State, Nigeria.
Batholomew Nzemua Ahmadu
Batholomew Nzemua Ahmadu
Department of Medical
Laboratory Science, Maryam Abacha American University of Niger, Maradi, Niger.
Ikenna Kingsley Uchendu
Ikenna Kingsley Uchendu
Corresponding
Author
Department of Medical Laboratory Science, University of Nigeria, Enugu Campus, Nigeria.
E-mail: uchenduikenna1@gmail.com
Abstract
The concurrent intake of herbal products (of similar
phytochemical components as fruits and vegetables) is one of the several
factors that can cause inter-patient variability in ARV drugs concentrations
and pharmacokinetics which may not be clinically favourable. This study
employed a cross-over design to investigate the effect of concurrent
consumption of mango fruit with Anti-retroviral drugs (zidovudine, lamivudine
and nevirapine). Sixteen (16) human subjects (8 Males, 8 Females) participated
in the study, and following a single dose administration per
day of
a fixed-dose tablet for one week, blood samples were collected at 1hr, 4hr and
12hr for the estimation of each drug using an HPLC analysis. Drug plasma
concentrations at baseline, after one week of concurrent Mango fruit
consumption and after another one week without Mango were estimated for each
subject. Statistical significance was evaluated using p-values generated
from a Student’s t-test at a C I
of 95% with a p-value of less or equal to 0.05. At 1 h, there was a significant decrease in the mean
plasma concentration of Lamivudine, dropping from 10.92 ± 2.91 to 7.91 ± 2.35 (Mean ±
SD) under the influence of Mango fruit. However, this and other
differences in zidovudine and nevirapine mean plasma concentrations
demonstrated across the groups are not statistically significant. Hence, the
concurrent consumption of Mango fruit and the administration of Anti-retroviral
drugs studied are not likely to produce any clinically significant negative
outcomes.
Abstract Keywords
Mangifera indica, anti-retroviral drugs, lamivudine, nevirapine, zidovudine
1. Introduction
It is a common sight to see people consume varieties of
fruits in considerable quantities especially at the peak of their seasons, and
people taking medications such as anti-retrovirals are not an exception to this
trend. The discovery that grape fruit juice inhibits Cytochrome P450 (CYP3A4)
in the wall of the small intestine [1, 2], raises concerns about other possible
interactions involving complex phytonutrients (in fruits, vegetables, herbs,
spices and teas) that might be of clinical importance. Complex phytonutrients
are known to have the greatest potential to induce or inhibit the activity of
drug metabolising enzymes which are thought to be highly expressed in the wall
of the small intestine [3]. The effect of grape fruit juice, apple,
pomegranate, guava and that of mango (Mangifera
indica) on commonly used medications such as statins, antihypertensives,
central nervous system modulators, immune suppressants, anti-histamines and
others have been described extensively [4].
Recent evidence has also documented the interaction between
St. John’s wort and certain antiretroviral drugs. St. John’s wort was found to
reduce plasma level of indinavir and that of a preparation of lopinavir/ritonavir
[5,
6].
Food/fruit–drug
interactions can result in two main clinical effects; decreased bioavailability
of a drug which predisposes to treatment failure or an increased
bioavailability which increases the risk of adverse events which may sometimes
be life threatening [4]. Such interactions are considered
clinically significant if they alter the expected therapeutic response. A lot
of research has been done on drug-drug interactions. However, only limited
studies have reported food/nutrient–drug
interaction with a number of these studies highlighting the effect of different
fruits and vegetables on intestinal absorption through interaction with drug
transporters as well as drug metabolising enzyme systems [4]. Studies investigating nutrient-ARV
interaction are not numerous but it has been widely established that
non–nucleoside reverse transcriptase inhibitors (NNRTIs) and protease
inhibitors (PIs) are substrates of the CYP450 enzyme system [7,
8].
Current reports indicated that the present
failure rate among patients receiving ARVs may be as high as 50% thereby
requiring the development of more tools such as therapeutic drug monitoring
(TDM) to ensure treatment efficacy [9]. Patients rarely report their
concomitant use of herbal products alongside their HAART medications much less
see any important correlation between their ARV medications and concurrent
intake of fruit or vegetable [10]. In fact, many physicians need more knowledge on this subject
matter to enable them to counsel their patients appropriately on possible
food/nutrient–ARV interaction which may not be clinically favourable [10]. The aim of this study is to investigate
the effect of consumption of Mango fruit on the concentration profile of
anti-retroviral drugs i.e. zidovudine, lamivudine and nevirapine.
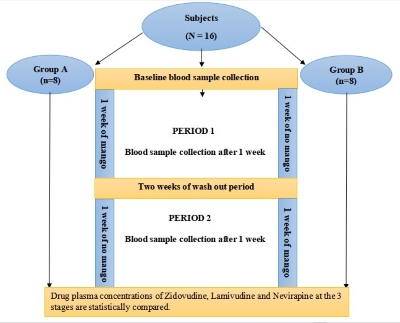
2.1 Study design
2.1.1 Study participants
Sixteen (16) human subjects comprising
of 8 males and 8 females (age 17-50 years and weighing 42-76 kg) were enrolled
for the study according to the United States Food and Drug
Administration document for conducting bioavailability and FED-bioequivalence
studies [11]. Computer-based
randomisation was used to allocate the subjects into two (2) groups of eight
persons per group, 4 males and 4
females (Decision Analyst STATSTM 2.0). Twelve (12) subjects were
recommended but 16 subjects were recruited for this study. A randomised, single
dose, one treatment, two periods cross-over design was used [12].
2.1.2
Inclusion and exclusion criteria
Any clinically stable
patient with CD4 count of 200 cells/mm3 or above, whose haemoglobin
level was at least 10.0 g/dl and who is comfortable with the dosage regimen to
be observed during the study was included. They were those who usually take
Mango fruit and would be able to tolerate the intended quantity for the
duration of the study (relying on their experience with mango consumption).
Patients currently taking any prescription or OTC medicine, pregnant women and
those who smoke or drink alcohol were excluded.
2.2 Reagents and chemical
Pure samples (Sigma-Aldrich,
Germany); Zidovudine pure sample (1 GM); Lamivudine pure sample (1 GM);
Nevirapine Pure sample (10 MG), Acetonitrile (HPLC grade); A Fixed dose
combination tablet containing zidovudine, lamivudine and nevirapine (300 mg,
150 mg, 200 mg) donated by the Pharmacy Department of the Antiretroviral Clinic
of Specialist Hospital Sokoto; A TDL-4 Centrifuge operated at 2000 rpm for 20
minutes was utilized and 0.45 micron Syringe filters were used for the study.
2.3 Ethical approval
Approval was obtained
from the Hospital Ethical Committee to carry out this study. Informed consent was obtained from subjects,
and the clinical research was conducted in accordance with the hospital ethical
committee’s guidelines for human experimentation.
The study participants were selected based on the earlier stated inclusion and
exclusion criteria and the subjects were educated on their involvement in the
study. A survey was conducted to
determine the species of mango fruit to use, this helped ensure uniformity
throughout the study, and species with the local name “Paparanda” was selected.
This species was selected because of its constant availability in the market at
the quantity that could support the study from start to finish. It is also the species
with the widest acceptability by the consumers. Each of the unknown samples was
run and from the peak and the lowest area (height) of the chromatogram, the
appropriate concentrations for the calibration curves were determined. The calibration curves for the HPLC quantification were obtained by
preparing serial concentrations (40,000 ng/ml, 4000 ng/ml, and 400 ng/ml) for lamivudine
and zidovudine, and (8,000 ng/ml, 800 ng/ml, and 80 ng/ml) for Nevirapine. Each
concentration was run in triplicates.
Prior to the commencement of the study, all
subjects were instructed to stay away from any form of medication (except their
HAART medications), fruits or vegetables 72 h, before the commencement of the
study and they all volunteered their consent and cooperation. Subjects were received into the site
of the study after an overnight fasting lasting at least 10 h, Water intake restriction was observed by all subjects
1 hour before the consumption of the two (2) average sized Mango fruits adopted as the standard meal
for this study. The study meal (Mango fruits) was administered 12 h, concurrently with,
and in accordance with the usual dosage regimen of the drug combination being
studied. The test drug was administered immediately
after the consumption of the Mango fruits. Food intake restriction was observed
until 4 h, after the administration of the test drug [11].
2.4.1 Baseline
The 16 subjects
were randomised into 2 groups of 8 subjects which are denoted as group A and group
B. A test dose of a fixed dosed combination tablet containing Zidovudine 300
mg, Lamivudine 150 mg, and nevirapine 200 mg was administered to each subject
on empty stomach after an overnight fasting of 10-12 h. Three (3) ml
of whole blood was collected at 1hr post dose, at 4hr and a few minutes before
(12 h), the evening
dose (trough). This marks the baseline data for all the subjects.
2.4.1.1 Period
1
Starting from
the following day, subjects in group A immediately commenced 1 week of mango
fruit consumption while those in group B took nothing. At
the end of the seventh (7) days period, blood samples were collected from both
groups as observed during Baseline sampling. The two (2) average sized Mango fruits adopted
as the standard meal for this study were consumed by the subjects 12 h, concurrently with, and in accordance with the usual dosage regimen of the
drug combination being studied).
2.4.2 Washout period
The two groups
were made to proceed on a two weeks wash-out break. During this, they took only
their ARV medications with the observance of fruit and vegetable restriction.
2.4.2.1 Period
2
At the end of
the two (2) weeks wash-out period, the subjects in group B commenced a one -
week of mango fruit consumption, at the end of which the final blood sample
collection took place for both groups in a pattern earlier established marking
the end of this phase of the study.
Figure 1: Diagrammatic Representation
of the Study Design
2.5 Methodologies
2.5.1 HPLC method development
An already validated method as per USP guideline for the
simultaneous determination of
lamivudine, zidovudine, and nevirapine was employed [13].
2.5.2 Preparation of mobile phase
A mobile phase consisting of a mixture of 0.015 M of Potassium dihydrogen ortho-phosphate
(PH 5.0) and acetonitrile in a ratio of 45:55 % v/v was prepared and degassed by
sonication before use.
2.5.3 Chromatographic conditions
A HITACHI HPLC instrument equipped with a Li Chrospher RP 18
(15 cm
by 4.6 mm) analytical column, an l-2130, an l- 2200 sample injector with a 20 uL loop and l-2420 UV –Visible detector
was employed for this analysis. EZ Chrome
Elite software was used for the quantification of peaks. A grant sonicator was
used to enhance the dissolution of the standard. A Fisher Scientific AR 10 PH
meter was used for PH reading.
2.5.4 Sample pre-treatment and extraction
Drugs were extracted from the plasma sample using a protein
precipitation technique [14]. Acetonitrile was selected as the
precipitating agent at ratios 1:3 of the plasma sample to the solvent. The
mixture was mixed thoroughly, vortexed at room temperature and centrifuged at
2000 rpm for 20 min. The clear supernatant liquid was decanted and filtered
through a 0.45 um syringe membrane filter [15].
2.5.5 Preparation of stock standard solution
Stock solution of 1 mg/ml (1,000,000 ng/ml) Lamivudine, Zidovudine and
Nevirapine were prepared separately by dissolving 20 mg of each standard drug with a small
quantity of methanol in a separate volumetric flask. The content was sonicated
for 15 minutes and then made up to volume with methanol. Working standard
solutions were prepared from the individual stock solution with the mobile
phase as the diluents.
2.5.5.1 Working standard solution 1
Lamivudine (40,000 ng/ml) = 40,000 x 25/100,000 = (1 ml of stock standard lamivudine in 25 ml volumetric flask and make up to
volume with the mobile phase).
Zidovudine (40,000 ng/ml) =40,000 x 25/100,000 = (1 ml of stock standard zidovudine in 25 ml volumetric flask and make up to
volume with the mobile phase).
Nevirapine (8,000 ng/ml) = 8,000 x 5/100,000 = (0.2 ml of
stock standard nevirapine in 25 ml volumetric flask and make up to
volume with the mobile phase.)
Therefore 1 ml each of lamivudine, zidovudine and 0.2 ml of nevirapine stock solution was
mixed in a 25 ml volumetric flask and made up to volume with the mobile
phase.
2.5.5.2 Working standard solution 2
A volume of 2.5 ml was pipetted from working
standard solution 1 into a 25 ml volumetric flask and made up to
volume with the mobile phase to give 4,000 ng/ml lamivudine, 4,000ng/ml zidovudine
and 800 ng/ml nevirapine standard concentrations.
2.5.5.3 Working standard solution 3
A volume of 2.5 ml was pipetted from working standard
solution 2 into a 25 ml volumetric flask and made up to volume with the mobile
phase to give 400 ng/ml lamivudine, 400 ng/ml zidovudine and 80 ng/ml nevirapine standard
concentrations.
2.6 Statistical analysis
A paired sample
student T-test was conducted, using a confidence interval (CI) of 95 % at a P-value
of less than 0.05, statistical significance or otherwise was determined between
various concentrations from different phases of the study. The null
hypothesis states that the concurrent consumption of Mango fruit has no statistically
significant effect on the concentration profile of the antiretroviral drugs.
3.1 Plasma concentration
of lamivudine; baseline (no treatment), period 1 and period 2
Fig.2a-2c Show
the Mean Concentration –Standard Deviation Plots for groups A and B at the Baseline,
Period 1 and Period 2.
3.1.1 Plasma concentration of
lamivudine
3.1.1.1 Baseline (no treatment in the
two groups)
At
the baseline, group B demonstrates a generally higher mean plasma concentration
than subjects in group A (Fig. 2a). At I h, group A has a mean
concentration of 10.62 ± 1.93 while group B has 10.92 ± 2.30. At 4 h, group A has a mean
concentration of 8.77 ± 2.56 while group B has 10.00 ± 2.68. At 12 h, group A has a mean plasma
concentration of 8.70 ± 2.41 while group B has 10.18 ± 2.55. However, all the differences in
mean plasma concentration observed were not statistically significant (P>0.05).
3.1.1.1.1 Period 1 (group A ‘with
Mango’ against group ‘No Mango’)
Across
all the three time points, group A subject present higher mean concentration than
the subjects in group B with the exception of the 1 h, time point where group A
has 7.91 ± 2.35 which is lower compared to 10.92 ± 2.91 presented by group B. But at 4 h, and
12 h, group A presents a mean concentration of 10.18 ± 3.34 and 9.69 ± 1.69 which are higher than 10.06 ± 2.26 and 9.18 ± 1.36 of group B respectively (Fig. 2b).
3.1.1.1.2 Period 2 (group A ‘No Mango’
against group B ‘with Mango’)
At
the Period 2, group B demonstrates a generally higher mean plasma concentration
than group A (Fig.2c). At I h, group A has a mean concentration of 11.79 ± 3.31 while group B has 124.21 ± 8.87. At 4 h, group A has a mean
concentration of 13.47 ± 5.54 while group B has 20.62 ± 8.03. At 12 h, group A has a mean plasma
concentration of 10.78 ± 2.05 while group B has 24.69 ± 7.77. It is worthy to highlight that
subjects in group B demonstrate higher mean concentration across the three time
points, and are statistically significant (P<0.05) at 1 and 12 h, following their
exposure to Mango fruit.
Figure 2a. Mean
Concentration-Standard deviation plot of Group A and Group B at Baseline (No
Treatment in the Two (2) groups).
Figure 2b. Mean
Concentration-Standard deviation plot of Group A and Group B at Period 1(Group A
‘with Mango’ against Group B ‘No Mango’).
Figure 2c. Mean
Concentration-Standard deviation plot of Group A and Group B at Period 2 (Group A
‘No Mango’ against Group B ‘with Mango).
3.2 Plasma concentration
of zidovudine; baseline (no treatment), period 1 and period 2
Fig. 3a-3c Show
the Mean Concentration –Standard Deviation Plots for group A and B at the baseline,
Period 1 and Period 2.
3.2.1 Plasma concentration of zidovudine
3.2.1.1 Baseline (no treatment in the
two groups)
Across
the three time points, group A demonstrates consistently higher mean plasma
concentrations than the group B. The mean concentration is 0.42 ± 0.28 at 1 h, in group A while group B
has 0.39 ± 0.13. At the 4 h, group B has only 0.13 ± 0.06 while group A has 0.28 ± 0.16. At the 12 h, group A records 0.22 ± 0.14 as against 0.14 ± 0.12 presented by the B group. On the overall, only the difference
between the groups at the 4 h, time point is statistically significant (P<0.05) while that of 1 h, and 12 h, are not
statistically significant (Fig.3a).
3.2.1.1.1 Period 1 (group A ‘with
Mango’ against group ‘No Mango’)
At
1 h, time point, in the presence of Mango, group A presents a lower mean
concentration of 0.34 ± 0.21 compared to the group with ‘No
Mango’, 0.50 ± 0.25. At 4 h, there is also a slight depression of the mean
plasma concentration with group A presenting 0.17 ± 0.10 compared to group B with ‘No
Mango’ with 0.20 ± 0.13. At 12 h, there was a reversal of the mean concentration
lowering trend as group A presents a higher concentration of 0.22 ± 0.17 as against 0.20 ± 0.21 of group B (Fig.3b). However, none of the differences is
statistically significant (P<0.05).
3.2.1.1.2 Period 2 (group A ‘No Mango’ against group B
‘with Mango’)
At
1 h, group B ‘with Mango’ demonstrates a higher mean plasma concentration of
0.35 ± 0.13 while group A ‘no Mango’ has 0.24 ± 0.12. At 4 h, group B ‘with Mango’ presents a
lower mean plasma concentration of 0.20 ± 0.16 compared to group A ‘No Mango’ which
presented a mean concentration of 0.26 ± 0.14. At 12 h, there is a slight rais in the
mean concentration of group B in the presence of Mango while group A, ‘No
Mango’ has 0.14 ± 0.13. But generally, all the differences reported were not
statistically significant (P>0.05) (Fig.3c).
Figure 3a. Mean
Concentration-Standard deviation plot of groups A and B at baseline (No treatment in the two groups)
Figure 3b. Mean
Concentration-Standard deviation plot of groups A and B at Period 1(Group A
‘with Mango’ against Group ‘No Mango’).
Figure 3c. Mean Concentration-Standard deviation
plot of group A and B at Period 2(Group A ‘No Mango’ against Group B
‘with Mango’).
3.3 Plasma concentration
of nevirapine: baseline (no treatment), period 1 and period 2
Fig.4a-4c Show
the Mean Concentration –Standard Deviation Plots for group A and B at the baseline,
Period 1 and Period 2.
3.3.1 Plasma concentration of
nevirapine
3.3.1.1 Baseline (no treatment in the
two groups)
At
1 h, group B shows a tendency for a lower mean plasma concentration of 5.88 ± 3.87 while group A has 10.43 ± 4.13. At 4 h, group A demonstrates a higher
mean concentration of 10.21 ± 6.11 as against group B that present 7.29 ± 2.97. At 12 h, group A has a slightly higher
mean concentration of 7.20 ± 4.81 as against group B with a mean
plasma concentration of 5.49 ± 3.85. The difference is statistically
significant (P<0.05) at 1 h, time point between group A and group B (Fig.4a).
3.3.1.1.1 Period 1 (group A ‘with
Mango’ against group B ‘No Mango’
At
1 h, the mean concentration of group A in the presence of Mango, 9.08 ± 5.36 is lower than 10.23 ± 5.37 recorded in group B. At the 4 h, time point, group A
presents a higher mean concentration of 11.60 ± 3.63 than 9.69 ± 6.22 presented by group B. At 12 h, group A still maintained a
higher mean plasma concentration of 10.43 ± 8.00 which is higher than 9.31 ± 5.55 in group B. However, the
differences across the groups are not statistically significant (P>0.05) (Fig.4b).
3.3.1.1.2 Period 2 (group A ‘No Mango’
against group B ‘with Mango’)
At
1 h, group B that consumed mango demonstrated a slightly lower mean
concentration of 9.67 ± 4.66 while group A did not take Mango had
10.68 ± 3.71. At 4 h, the Mango treated group B has a higher mean
concentration of 14.52 ± 11.70 while group A without Mango, has
10.69 ± 4.80. At 12 h, the Mango treated group B showed
a lower mean concentration of 7.07 ± 6.7 while group A, without Mango
demonstrates a higher mean plasma concentration of 11.46 ± 3.57.However,all the
reported differences are not statistically significant (P>0.05) across all three time points
(Fig.4c)
Figure 4a. Mean
Concentration-Standard deviation plot of groups A and B at baseline (No treatment
in the two groups)
Figure 4b. Mean
Concentration-Standard deviation plot of groups A and B at Period 1 (Group A
‘with Mango’ against Group B ‘No Mango’)
Figure 4c. Mean
Concentration-Standard deviation plot of groups A and B at Period 2 (Group A ‘No
Mango’ against Group B ‘with Mango’)
4.
Discussion
Specific foods
or drinks can affect how a medicine is absorbed, metabolised, or utilised by
the body. This is referred to as a drug-food interaction. These interactions
may alter how effectively a treatment works or have unintended consequences [16, 17]. Numerous
medications, including statins (used to lower cholesterol), blood pressure
medications, and psychiatric medications, have been shown to interact with
grapefruit juice. It may impair the function of gastrointestinal enzymes, elevating
medication levels in the blood and increasing the risk of toxicity or bad
effects [18, 19].
The drugs
zidovudine, lamivudine, and nevirapine are all used to treat HIV (Human
Immunodeficiency Virus) infection. These medications are often administered as
part of a combination antiretroviral therapy (ART) regimen to manage the virus
and reduce the progression of HIV to AIDS (Acquired Immunodeficiency Syndrome) [20].
Our study investigated the effect of concurrent consumption
of mango fruit on the concentration profile of patients on Highly Active
Anti-retroviral Therapy (HAART) medications namely; Lamivudine, Zidovudine and
Nevirapine.
For lamivudine, at
the baseline (no treatment phase) there is a tendency for the mean plasma
concentrations in group B to be higher than those of group A, but the
differences demonstrated are not statistically significant across the three
time points of the study. The groups are therefore considered balanced. This suggests that the randomization
done at the beginning of this study was adequate and any difference observed between
groups A and B going further in this study will be significant and are not
biased by baseline differences.
At Period 1, there was a statistically significant (P<0.05) difference in the 1 h, mean plasma
concentration with group B (No Mango) having 10.92 ± 2.91 tending to be higher than group A
(With mango) with 7.91 ± 2.35 mean plasma concentration. Taken
alone, this suggests Mango lowers the plasma concentration of Lamivudine at 1 h.
However, in Period 2, this trend was neither altered nor reversed even after
the group B subjects were exposed to mango fruit for I week. In essence, if
Mango fruit was responsible for lower mean concentrations in group A when
exposed to mango in Period1 it should have done so in group B (With Mango) in
Period 2. Instead, group B (with mango) in period 2 tends to present a
significantly higher (P<0.05) mean value of 24.21 ± 8.87 at 1 h, time point when compared
with group A (No Mango) having 11.79 ± 3.31 mean concentration. Since the
plasma concentration suppression effect did not move with the mango in period 2
as observed in period 1, apparently, the presence of mango fruit cannot be
considered responsible for the mean concentration value lowering i.e. other
factors other than the mango effect might have been responsible for our
observation. In vitro study by Rodeiro et
al. [21], suggests that Mango and its components
lower the activities of CYP 450 enzymes, but it has also been established that
there is little or no possibility of Lamivudine interaction with drug or herbal
(phytocomponents) sharing the CYP 450 metabolic pathway due to low extent of
metabolism and low protein binding, hence justifying our observation that mango
fruit does not alter the concentration profile of Lamivudine.
For Zidovudine
and Nevirapine, the study did not
demonstrate any statistically significant difference in the mean plasma
concentrations of Zidovudine and Nevirapine across all the three phases of this
study. This suggests mango fruit may affect the concentration profile of zidovudine
and nevirapine. This could be because zidovudine and nevirapine are easily absorbed
through the gastrointestinal tract. Food can influence zidovudine absorption.
Taking zidovudine with a meal, particularly a high-fat meal, can reduce absorption.
For
optimum absorption, zidovudine is recommended to be taken on an empty stomach.
Nevirapine, unlike zidovudine, can be taken with or without food because food does
not affect its absorption [22].
5. Conclusions and limitation
The results of our study show that the concentration profile
of the subjects with respect to the ARV medications (Lamivudine150mg,
Zidovudine 300mg, and Nevirapine 200mg) were not significantly altered in the
presence of mango fruit. Therefore, patients taking Mango fruit with these
medications are not likely to experience any negative or unfavourable food-drug
interaction that can be of any clinical importance.
The design is deliberately ‘data poor technique’ for ethical
reasons as the subjects are ill patients with active Human Immunodeficiency
Virus (HIV) and may not be able to withstand too many blood samples. Also, the
study was done on patients who have been on these medications for varying
length of time, and are already at their steady state concentrations, and it
was considered ethically inappropriate to stop them temporarily for the main
purpose of carrying out proper pharmacokinetic profiling. The design was
therefore targeted at the trough concentrations which were the concentrations
just before the next dose.
ADME
–Absorption-Distribution-Metabolism-Elimination.
ART-Anti-retroviral therapy
ARVs-Anti-Retrovirals
AUC –Area Under the Curve
CI –Confidence Interval
C Max-Maximum Plasma Concentration
FDA- Food and Drug Administration
HAART - Highly Active Anti-Retroviral
Therapy.
HIV/AIDS-Human Immunodeficiency Syndrome
HPLC-UV-High Performance Liquid
Chromatography-Ultra Violet
MTCT-Mother-to Child-Transmission
NNRTI–Non-nucleoside Reverse
Transcriptase Enzyme Inhibitor
NRTIs–Nucleoside Reverse Transcriptase
Enzyme Inhibitor
OTC –Over the Counter
PK-Pharmacokinetic
TDM-Therapeutic Drug Monitoring
T Max-Time to Maximum Concentration
WHO-World Health Organization
Authors’
contributions
All the authors contributed equally to writing the content.
Acknowledgements
None
Funding
This work was not financed by any agency other than
the authors.
Availability of data and materials
All
relevant data are within the paper and its supporting information files. Additional data will
be made available on request according to the journal policy.
Conflicts
of interest
The authors have no conflicts of interest to
declare.
References
1.
Sikma, M.A.; Van Maarseveen, E.M.; Van De Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Donker, D.W.; Kesecioglu, J.; Meulenbelt, J. Pharmacokinetics and toxicity of tacrolimus early after
heart and lung transplantation. Am. J. Transplant. 2015, 15(9), 2301-2313.
2.
Prakash, C.; Zuniga, B.; Song, C.S.; Jiang, S.; Cropper, J.; Park, S.; Chatterjee, B. Nuclear receptors in drug metabolism, drug response and
drug interactions. Nucl. Recept. Res. 2015, 2,
101178.
3.
Boronat, A.; Rodriguez-Morató, J.; Serreli, G.; Fitó. M.; Tyndale, R.; Deiana, M.; de la Torre, R. Contribution of biotransformations carried out by the
microbiota, drug-metabolizing enzymes, and transport proteins to the biological
activities of phytochemicals found in the diet. Adv. Nutr. 2021, 12(6), 2172-2189.
4.
Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and
Vegetables-the Millennium’s Health. Int. J. Food Sci. Technol. 2008, 36 (7), 703-725.
5.
Steenkamp, V.; Parkar, H.; Dasgupta, A. Utility of Therapeutic Drug Monitoring in Identifying
Clinically Significant Interactions Between St. John's Wort and Prescription
Drugs. Ther. Drug Monit. 2023, 45(1), 35-44.
6.
Jalloh, M.A; Gregory, P.J.; Hein, D.; Risoldi Cochrane, Z.; Rodriguez, A. Dietary supplement interactions with antiretrovirals: a
systematic review. Int. J. STD AIDS. 2017, 28(1), 4-15.
7.
Usman, S.O.; Agbaje, E.O.; Oreagba, I.A.; Akanmu, A.S. Pharmacokinetic Interactions between
ArtemetherlLnmefantrine and Commonly used Antiretroviral Drugs: A Review. Uni. Lag. J.
Basic Med. Sci. 2022, 4(8).
8.
Adkison, K.; Wolstenholme, A.; Lou, Y.; Zhang, Z.; Eld, A.; Perger, T.; Vangerow, H.; Hayward, K.; Shaefer, M.; McCoig, C. Effect of sorbitol on the pharmacokinetic profile of
lamivudine oral solution in adults: an open‐label, randomized study. Clin. Pharmacol. Ther. 2018, 103(3), 402-408.
9.
Pretorius, E.; Klinker, H.; Rosenkranz, B. The role of therapeutic
drug monitoring in the management of patients with human immunodeficiency virus
infection. Ther. Drug Monit. 2011, 33(3), 265-274.
10.
Bent, S. Herbal medicine in United State: Review of
Efficacy,safety,and regulation.J. Gen.
Intern. Med. 2008, 23(6), 854–859.
11.
Li, M.; Zhao, P.; Pan, Y.; Wagner, C. Predictive performance of physiologically based
pharmacokinetic models for the effect of food on oral drug absorption: current
status. CPT: Pharmacomet Syst Pharmacol. 2018, 7(2), 82-89.
12.
Piscitelli, S.C.; Gallicano, K.D. Interactions among drugs for HIV and
opportunistic infections. New Engl. J. Med. 2001, 344, 984-996.
13.
Anantha, K.D.; Naveen Babu, M.V.; Seshagiri Rao, J.V.L.N.; Jayathirtha Rao,V. Simultaneous determination
of lamivudine,Zidovudine and nevirapine in tablet dosage forms by the RP-HPLC
method. Rasayan J. Chem. 2010, 3(1), 94-99.
14.
Alebouyeh, M.; Amini, H. Rapid determination of lamivudine in human plasma by
high-performance liquid chromatography. J. Chromatogr. B. 2015, 975, 40-44.
15.
Kabra, V.; Agrahari, V.; Karthikeyan, C.; Trivedi, P. Simultaneous quantitative determination of zidovudine and
nevirapine in human plasma using isocratic, reverse phase high performance
liquid chromatography. Trop. J.
Pharm. Res. 2009, 8(1), 79-86.
16.
D’Alessandro, C.; Benedetti, A.; Di Paolo, A; Giannese, D.; Cupisti, A. Interactions between food and drugs, and nutritional
status in renal patients: a narrative review. Nutrients. 2022, 14(1), 212.
17.
Mahata, P.P.; Thakur, A.; Chakraborty, S.; Bala, N.N. A review on pharmacist's role in mitigation of food-drug interactions.
Res. J. Pharm. Technol. 2015, 8(4), 423-31.
18. Day, R.O.; Snowden, L.; McLachlan, A.J. Life‐threatening drug interactions: what the physician
needs to know. Intern. Med. J. 2017, 47(5), 501-12.
19. Mallhi TH, Sarriff A, Adnan AS, Khan YH, Qadir MI, Hamzah AA,
Khan AH. Effect of fruit/vegetable-drug interactions on CYP450, OATP and
p-glycoprotein: A systematic review. Trop. J. Pharm. Res. 2015, 14(10), 1927-35.
20. Nashid, N.; Khan, S.; Loutfy, M.; MacGillivra, J.; Yudin,
M.H.; Campbell, D.M.; Barozzino, T.; Baqi, M.; Read, S.E., Bitnun, A. Breastfeeding by women living with human immunodeficiency
virus in a resource-rich setting: a case series of maternal and infant
management and outcomes. J. Pediatric. Infect. Dis. Soc. 2020, 9(2), 228-31.
21. Rodeiro, I.; Donato, M.T.; Lahoz, A.; Garrido, G.; Delgado, R.; Gomez-Lechon, M.J. Interactions of polyphenols with the P450 system: possible implications for human therapeutics. Mini-Rev. Med. Chem. 2008, 8, 97–106.
22. Siritientong, T.; Thet, D.; Methaneethorn, J.; Leelakanok, N. Pharmacokinetic outcomes of the interactions of antiretroviral agents with food and supplements: a systematic review and meta-analysis. Nutrients. 2022, 14(3), 520.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
The concurrent intake of herbal products (of similar
phytochemical components as fruits and vegetables) is one of the several
factors that can cause inter-patient variability in ARV drugs concentrations
and pharmacokinetics which may not be clinically favourable. This study
employed a cross-over design to investigate the effect of concurrent
consumption of mango fruit with Anti-retroviral drugs (zidovudine, lamivudine
and nevirapine). Sixteen (16) human subjects (8 Males, 8 Females) participated
in the study, and following a single dose administration per
day of
a fixed-dose tablet for one week, blood samples were collected at 1hr, 4hr and
12hr for the estimation of each drug using an HPLC analysis. Drug plasma
concentrations at baseline, after one week of concurrent Mango fruit
consumption and after another one week without Mango were estimated for each
subject. Statistical significance was evaluated using p-values generated
from a Student’s t-test at a C I
of 95% with a p-value of less or equal to 0.05. At 1 h, there was a significant decrease in the mean
plasma concentration of Lamivudine, dropping from 10.92 ± 2.91 to 7.91 ± 2.35 (Mean ±
SD) under the influence of Mango fruit. However, this and other
differences in zidovudine and nevirapine mean plasma concentrations
demonstrated across the groups are not statistically significant. Hence, the
concurrent consumption of Mango fruit and the administration of Anti-retroviral
drugs studied are not likely to produce any clinically significant negative
outcomes.
Abstract Keywords
Mangifera indica, anti-retroviral drugs, lamivudine, nevirapine, zidovudine
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


