Research Article
Tyler M. Wilson*
Tyler M. Wilson*
Corresponding
Author
D. Gary Young
Research Institute, Lehi, UT 84043, USA.
E-mail: tywilson@youngliving.com; Tel.: +1-801-669-4501
Thomas T. McKay
Thomas T. McKay
D. Gary Young
Research Institute, Lehi, UT 84043, USA.
Hsueh-Kung Lin
Hsueh-Kung Lin
D. Gary Young Research Institute, Lehi, UT 84043, USA.
Chris Packer
Chris Packer
D. Gary Young Research Institute, Lehi, UT 84043, USA.
Christopher R. Bowerbank
Christopher R. Bowerbank
D. Gary Young Research Institute, Lehi, UT 84043, USA.
Received: 2025-06-10 | Revised:2025-06-24 | Accepted: 2025-06-27 | Published: 2025-06-30
Pages: 60-68
DOI: https://doi.org/10.58985/jeopc.2025.v03i01.66
Abstract
Essential oils (EO), sometimes called volatile oils, are produced by aromatic plants and extracted from various plant parts. EO have historically been extracted using various solvents, with water likely the most common solvent. EO are largely composed of volatile compounds, such as phenylpropanoids, monoterpenoids, sesquiterpenoids, and to a lesser extent, diterpenoids. The current study investigates a novel, patents pending extraction technique that uses EO as a natural solvent to extract additional, non-volatile compounds from plant materials that were otherwise devoid from EO. While the application is seemingly endless with various plant materials, the current study focuses on (1) using frankincense (Boswellia sacra) resin to produce an EO through hydrodistillation and then (2) using said EO as a solvent on the ‘spent’ frankincense resin to extract additional, non-volatile compounds. From the initial extraction, frankincense EO samples were largely comprised (determined by GC/MS) of α-pinene (avg. 39.8%), limonene (avg. 16.4%), and contained detectable compounds as large as α-phellandrene dimer (C20H32), albeit in trace amounts. From the secondary extraction, where EO was used as a solvent on spent frankincense resin, the volatile profile was similar, but additional non-volatile compounds were detected (determined by LC/MS/MS) such as α-boswellic acid (C30H48O3) (avg. 178.92 μg/mL), 3-acetyl-11-keto-β-boswellic acid (C32H48O5) (avg. 40.40 μg/mL), and 11-keto-β-boswellic acid (C30H46O4) (avg. 13.87 μg/mL). The current study establishes a novel and sustainable extraction technique that has potential applications across multiple industries, including the flavor and fragrance, and pharmaceutical industries.
Keywords
Boswellia sacra, boswellic acids, frankincense, monoterpenoids; resin, triterpenoids.
1. Introduction
Boswellia sacra
Flück (frankincense) is a deciduous tree belonging to the Burseraceae family [1]. Frankincense trees naturally produce aromatic
resins, however, the trees are typically incised to induce additional resin
exudation [2]. The exuded and dried resin is
then collected for a variety of uses, among which include hydrodistillation for
the production of frankincense essential oil (EO).
Boswellia sacra
EO is largely composed of monoterpenes, sesquiterpenes (to a lesser degree),
and is typically devoid of larger compounds such as diterpenes and triterpenes [3]. Frankincense (B. sacra) EO has demonstrated
antimicrobial properties against various pathogens [4].
Additionally, frankincense EO has purported utility as an oncological
tool [5, 6]. In a study by Ni and associates
[7], fractions of frankincense EO, resulting
from an extended period of extraction, that contained detectable quantities of
boswellic acids (triterpenoids) demonstrated more bioactivity than fractions
devoid of triterpenoids.
Triterpenoids,
such as boswellic acids, are not typically recovered from hydrodistilled
frankincense EO due to their relatively large molecular weights. Instead,
boswellic acids are extracted from frankincense resin using solvents such as
dichloromethane or methanol [8, 9]. Boswellic
acids have demonstrated antiparasitic and antimicrobial properties [8,10] and have purported utility in the medical
industry for oncological, antidiabetic, anti-inflammatory, and antidepressant ingredients
[9,12-14]. The literature suggests that
while frankincense EO has many biological functions, the triterpenoids which
are typically not recovered in the EO have a broader utility and their own
increased biological functions.
The current study uses a novel, patents pending extraction technique [15] that uses hydrodistilled frankincense EO as a secondary solvent to then extract triterpenoids from frankincense resin. This approach eliminates the use of harsh chemical solvents (dichloromethane, methanol, etc.) and, since the secondary extraction is conducted on spent frankincense resin, establishes an environmentally sustainable approach to obtain the sought-after triterpenoids. The current study establishes the different chemical profiles of frankincense essential oils (n = 3) and secondary extracted (DeepSpectra® extraction) samples (n = 3) by GC/MS analysis. Consistent with previous research, frankincense essential oils were largely composed of volatile compounds (monoterpenoids and sesquiterpenoids). DeepSpectra® samples, which also contained a similar volatile profile, were additionally comprised of a non-volatile fraction (triterpenoids), which was determined by LC/MS/MS. While said patents pending technology [15] has a seemingly endless list of applications, the current study demonstrates its application and utility with a single plant material, frankincense resin.
2. Materials and
methods
2.1.
Distillation and extraction techniques
Frankincense (Boswellia sacra) resin was procured from the Federal Republic of Somalia (Albert Vieille, Vallauris, France) (Fig. 1). The resin was procured in 2024 and delivered in a dried/hardened state.
Figure 1. Raw frankincense
(Boswellia sacra) resin prior to
hydrodistillation.
Essential oil (EO) samples (n = 3) were produced by laboratory-scale hydrodistillation as follows: 3 L of water was added to the bottom of a 12-L distillation chamber (Albrigi Luigi S.R.L., Grezzana, Italy) and approximately 1 kg of resin was accurately weighed and added to the distillation chamber. Hydrodistillation was performed for 3 h, and volatile oil was separated using a cooled condenser and Florentine flask. The essential oil samples were filtered and stored in a sealed amber glass bottle at room temperature until use for secondary extraction or analysis. The frankincense resin that no longer had EO (spent resin) was separated from any remaining water, allowed to dry at room temperature for 72 h, and broken into small (approx. 3 cm x 3 cm) pieces (Fig. 2).
Figure 2. Spent, dried frankincense
(Boswellia sacra) resin following
hydrodistillation.
Secondary extraction DeepSpectra® samples (n = 3) were produced as follows: Dried pieces of spent resin were ground to a particle size #18 (1000 microns) using a mortar and pestle and an ASTM E-11 USA Standard Sieve (Dual Manufacturing Co., Inc., Franklin Park, IL, USA) (Fig. 3), accurately weighed and added to EO (approx. 1:3), mixed in a beaker at 300 rpm for 3 h, and filtered using a 0.22 μm PVDF Luer lock filter (Restek Corporation, Bellefonte, PA, USA). DeepSpectra® samples (n = 3) were derived from the respective EO samples and spent materials (i.e., DeepSpectra® sample A produced by mixing EO sample A with spent resin from EO sample A hydrodistillation, etc.). The DeepSpectra® sample extraction details are presented in Table 1.
Figure 3. Spent, dried, and ground
frankincense (Boswellia sacra)
resin for secondary extraction
DeepSpectra® samples.
Table 1. Secondary extraction, or DeepSpectra® (DS) extraction, details including spent resin mass (g) and essential oil mass (g) used for production of each sample.
Samples | DS Sample A | DS Sample B | DS Sample C |
Spent Resin Mass (g) | 7.59 | 8.65 | 7.05 |
Essential Oil Mass (g) | 22.77 | 25.78 | 21.14 |
2.2. Analysis methods
Relative density (specific gravity) analysis was conducted using a density meter (Anton Paar, Graz, Austria) in accordance with the International Organization for Standardization’s (ISO) 279 [16].
To determine volatile compound profiles, EO and DeepSpectra® samples were analyzed, and compounds were identified and quantified by GC/MS using an Agilent 7890B GC/5977B MSD (Agilent Technologies, Santa Clara, CA, USA) and Agilent J & W DB-5, 60 m × 0.25 mm, 0.25 μm film thickness, fused silica capillary column. Operating conditions: 0.1 μL of sample (20% soln. for essential oils in ethanol), 100:1 split ratio, initial oven temp. of 40 °C with an initial hold time of 5 min, and oven ramp rate of 4.5 °C per min to 310 °C with a hold time of 5 min. The electron ionization energy was 70 eV, scan range was 35–650 amu, scan rate was 2.4 scans per s, source temp. 230 °C, and quadrupole temp. 150 °C. The compounds were identified using the Adams volatile oil library [17] and a Chemstation library search in conjunction with retention indices. Note that limonene/1,8-cineole co-elutes and their amounts were determined via the ratio of masses 68 and 93 (limonene), and 81 and 108 (1,8-cineole).
To determine the non-volatile compound profiles, EO and DeepSpectra® samples were analyzed by LC/MS/MS. Samples were prepared for analysis by adding 1.0 mL of HPLC grade ethanol (Sigma-Aldrich, 200 proof, item 459828) to approximately 100 mg of sample weighed into a standard HPLC vial and vortexed for approximately 30 seconds. Each sample was then filtered (Millex syringe filter, PTFE, 0.2 µm x 13 mm) into a second HPLC vial and analyzed for boswellic acid content by LC/MS/MS using an Agilent 6470 LC/TQ in dMRM negative ion mode attached to an Agilent 1290 Infinity II UPLC system (Agilent Technologies, Santa Clara, CA, USA). Analyte separation was achieved using an Agilent Zorbax Eclipse Plus C18 column (2.1 x 150 mm, 1.8 µm) under the following operating conditions: 5.0 µL of sample was injected onto the column and subjected to a 26-minute mobile phase and flow rate gradient (Table 2).
Table 2. Mobile phase gradient details include method time, flow rate, and concentrations of mobile phase A and mobile phase B.
Number | Time (min) | Flow (mL/min) | A (%) | B (%) |
1 | initial | 0.4 | 95 | 5 |
2 | 3.00 | 0.4 | 70 | 30 |
3 | 9.00 | 0.4 | 15 | 85 |
4 | 14.00 | 0.25 | 5 | 95 |
5 | 23.00 | 0.25 | 5 | 95 |
6 | 23.01 | 0.4 | 95 | 5 |
7 | 26.00 | 0.4 | 95 | 5 |
Mobile phase A (MPA) was 5 mM ammonium formate (Fisher, Optima LC/MS grade, item A115) in ultra-pure water (UPW), produced in-house using a Milli-Q IQ 7000 filtering system equipped with a Millipak 0.22 µm filter, and 0.1% formic acid (Fisher, Optima LC/MS grade, item A113). Mobile phase B (MPB) was 5 mM ammonium formate in methanol (HiPerSolv Chromanorm, LC/MS grade, VWR item BDH85800), with 0.1% formic acid. The column temperature was 40⁰C. Positive identification was achieved by both retention time comparison and specific molecular mass parent/product ion SRM transitions. Quantitation of each analyte was achieved by comparing peak area responses to an established calibration curve (quadratic regression, weighted 1/x, minimum R2 value of 0.995) with a range of 0.5 to 10 ug/mL (ppm). When necessary, samples were diluted in order to obtain peak responses within the calibration range. Primary SRM transitions for each analyte were as follows: 11-keto-beta-boswellic acid (KBA) (469.3→407.2), aceto-11-ketoboswellic acid (AKBA) (511.4→59.0), alpha-boswellic acid (455.4→437.4), beta-boswellic acid (455.4→437.4), acetyl-alpha-boswellic acid (497.4→59.0), acetyl-beta-boswellic acid (497.4→59.0). The fragmentor and collision energy voltages varied and were specific to each SRM transition. Calibration curves and retention times were established by using certified reference materials (ChromaDex, Boswellic Acids Frankincense Kit, Item KIT-00002605).
An additional analysis of the non-volatile compound profiles was conducted by Revident LC/QTOF. The separation conditions used for LC/QTOF were identical to those of the LC/MS/MS instrument conditions described above. Data were acquired between 40-1000 m/z at a rate of 1 spectra/sec.
3. Results
Hydrodistillation and production of frankincense (Boswellia sacra) essential oil (EO) samples A-C resulted in relatively consistent yields, ranging from 3.3-3.6% (w/w). The hydrodistillation details are presented in Table 3. The color and appearance of all EO samples were light-yellow and clear liquids.
Table 3. Hydrodistillation and essential oil (EO) production details include fresh resin mass (g), essential oil yield (g), and essential oil % (w/w).
Samples | EO Sample A | EO Sample B | EO Sample C |
Resin Mass (g) | 930.55 | 1037.40 | 926.65 |
EO Yield (g) | 28.46 | 31.59 | 27.02 |
EO % (w/w) | 3.5 | 3.6 | 3.3 |
The details of the DeepSpectra® extraction are presented in Table 1. When measuring pre- and post-weights (EO, DeepSpectra® oil) samples, there was a 15%+ increase in weight in the DeepSpectra® samples. However, trivial amounts of samples were lost in the filtering process; so, an accurately calculated increase in mass was not feasible and is not recorded in the manuscript. The color and appearance of all DeepSpectra® samples were yellow and clear liquids.
As an initial check on sample characteristics and differences, the specific gravity was measured for the initial extraction (essential oils) and DeepSpectra® samples. The results are presented in Table 4. Given the increased mass and specific gravity resulting from each secondary extraction, the data suggest that the EO is a reliable solvent for extracting additional compounds of higher molecular weight from the spent resin.
Table 4. Specific gravity values for essential oil (EO) and DeepSpectra® (DS) samples.
Samples | Frankincense EO | Frankincense DS |
Sample A | 0.87 | 0.91 |
Sample B | 0.87 | 0.91 |
Sample C | 0.87 | 0.91 |
A total of 49 volatile compounds were identified by GC/MS analysis (Supplementary Table S1 for the complete dataset). A summary of the GC findings is provided in Table 5, including all volatile compounds detected at ≥ 0.5% in at least one sample, which included 22 volatile compounds. Between associated samples (i.e., EO sample A and DeepSpectra® sample A, etc.), there were seemingly small differences in volatile profiles. The standard deviations of compounds between associated samples were all ≤ 0.1, with the exception of limonene (σ = 0.2 in sample group B, σ = 0.4 in sample group C), (E)-caryophyllene (σ = 0.2 in sample group C), and isocembrol (σ = 0.2 in sample group A, σ = 0.2 in sample group B, σ = 0.3 in sample group C). The volatile profiles were seemingly unchanged from the initial extraction (EO) and DeepSpectra® extraction. Isocembrol may be the exception as values displayed a higher standard deviation for all three sample groups and could be, as a diterpene alcohol (C20H34O), considered a semi-volatile aromatic compound that is most efficiently extracted during the secondary extraction process.
Table 5. Volatile compounds detected (≥ 0.5%) in at least one essential oil (EO) or DeepSpectra® (DS) sample.
Compound Name | KI | Frankincense EO (area %) | Frankincense DS (area %) | ||||
A | B | C | A | B | C | ||
α-Thujene | 924 | 5.2 | 5.3 | 5.1 | 5.2 | 5.1 | 5.1 |
α-Pinene | 932 | 38.9 | 41.2 | 39.3 | 38.9 | 40.8 | 40.1 |
Camphene | 946 | 0.9 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 |
Thuja-2,4(10)-diene | 953 | 0.4 | 0.5 | 0.5 | 0.4 | 0.5 | 0.5 |
Sabinene | 969 | 4.4 | 4.0 | 4.2 | 4.3 | 3.9 | 4.1 |
β-Pinene | 974 | 1.2 | 1.3 | 1.3 | 1.2 | 1.3 | 1.2 |
Myrcene | 988 | 6.1 | 6.0 | 5.1 | 5.9 | 5.8 | 4.9 |
α-Phellandrene | 1002 | 1.8 | 1.6 | 1.7 | 1.8 | 1.6 | 1.6 |
p-Cymene | 1020 | 5.5 | 5.7 | 6.0 | 5.5 | 5.6 | 6.0 |
Limonene | 1024 | 17.3 | 16.4 | 15.5 | 17.1 | 16.2 | 15.6 |
(E)-Pinocarveol | 1135 | 0.4 | 0.4 | 0.5 | 0.3 | 0.5 | 0.5 |
(E)-Verbenol | 1140 | 1.1 | 1.2 | 1.5 | 1.0 | 1.2 | 1.4 |
Verbenone | 1204 | 0.4 | 0.5 | 0.5 | 0.4 | 0.5 | 0.6 |
α-Copaene | 1374 | 1.7 | 1.6 | 1.5 | 1.6 | 1.6 | 1.4 |
β-Elemene | 1389 | 0.6 | 0.6 | 0.7 | 0.6 | 0.6 | 0.6 |
(E)-Caryophyllene | 1417 | 4.8 | 4.2 | 4.6 | 4.7 | 4.2 | 4.2 |
α-Humulene | 1452 | 1.2 | 1.0 | 1.1 | 1.2 | 1.0 | 0.9 |
α-Muurolene | 1500 | 0.5 | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 |
Cubebol | 1514 | 0.9 | 0.7 | 1.0 | 0.9 | 0.9 | 1.0 |
δ-Cadinene | 1522 | 1.2 | 1.1 | 1.2 | 1.2 | 1.1 | 1.1 |
Caryophyllene oxide | 1582 | 0.6 | 0.6 | 0.9 | 0.7 | 0.7 | 0.9 |
Isocembrol | *2094 | tr | tr | tr | 0.4 | 0.4 | 0.5 |
Total | 95.1 | 95.0 | 93.6 | 94.8 | 94.4 | 93.7 | |
Three frankincense essential oil (EO) samples (A-C) and three frankincense DeepSpectra® (DS) samples (A-C) were analyzed by GC/MS. The compound name, KI, and relative area % are reported. KI is the Kovat’s Index value and was previously calculated by Robert Adams using a linear calculation on a DB-5 column [17]. * KI manually calculated. | |||||||
Given the limited availability of commercially available reference standards, a total of six non-volatile compounds were identified by LC/MS/MS analysis. A summary of the LC findings is provided in Table 6. While all six non-volatile terpenoids were detected in each DeepSpectra® sample, none were detected in any of the EO samples.
Table 6. Non-volatile compounds detected in DeepSpectra® samples
Compound Name | Frankincense DS (μg/mL) | ||
A | B | C | |
α-Boswellic acid | 163.25 | 186.23 | 187.27 |
β-Boswellic acid | 734.35 | 872.72 | 872.64 |
3-Acetyl-11-keto-β-boswellic acid | 35.89 | 42.17 | 43.14 |
11-keto-β-boswellic acid | 11.12 | 14.85 | 15.63 |
Acetyl-α-boswellic acid | 414.80 | 496.87 | 483.96 |
Acetyl-β-boswellic acid | 1183.26 | 1275.36 | 1299.53 |
Compounds were measured in μg/mL. None of the above mentioned non-volatile compounds were detected in the frankincense essential oil samples (LOD 1 μg/mL). | |||
To investigate whether these six non-volatile compounds are the extent of extractable compounds that distinguish frankincense EO from DeepSpectra® samples, Revident LC/QTOF analysis was also performed. While confirmatory analysis of additional (other than the six aforementioned boswellic acids) and specific compounds was not conducted due to limited reference standards, a volcano plot was produced to display the differences between sample groups (Fig. 4).
Figure 4. Scatter plot (volcano plot) displaying the relationship between the sample groups, frankincense essential oil and frankincense DeepSpectra® samples. Red indications are upregulated in the DeepSpectra® samples as compared to the essential oil samples. Dark blue indications are down regulated in the DeepSpectra® samples compared to the essential oil samples. Light blue, yellow, and gray indications did not meet the cutoff requirements (change requirement = 2; p < 0.05).
4. Discussion
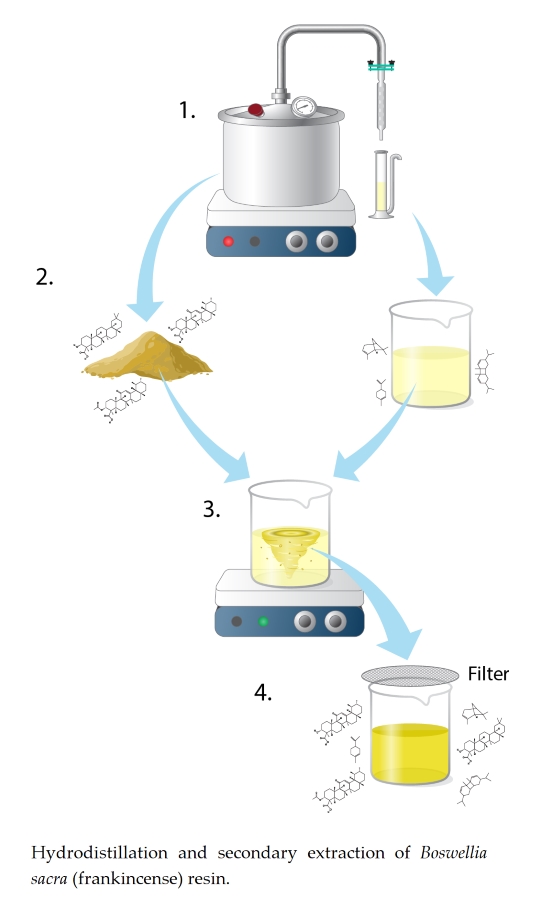
Plant resins are defined as being composed of both volatile and non-volatile fractions [18]. Additionally, resins typically contain small particulates or contaminates (tree bark, twigs, leaf materials, etc.) [19, 20]. Prior to conducting specific gravity measurements, or other analytical testing, samples were filtered as a final step in the DeepSpectra® extraction production process (Fig. 5). Filtration was conducted to remove any non-volatile debris that was not soluble in Boswellia sacra (frankincense) essential oil (EO), and to remove any aforementioned organic debris (Fig. 5).
Figure 5. Illustration of the hydrodistillation and secondary extraction (DeepSpectra® extraction) of Boswellia sacra (frankincense) resin. (1) Hydrodistillation and production of essential oil sample, (2) recovery of spent frankincense resin (left) and production of essential oil (right), (3) recombining and mixing of spent frankincense resin and essential oil for production of (4) DeepSpectra® oil. The compounds characteristic of the spent resin (step 2 left) and essential oil (step 2 right) were found in the final product (step 4). Illustrated by Rick Simonson, Science Lab Studios, Inc. (Kearney, NE, USA).
Upon hydrodistillation of frankincense resin and production of both the EO and DeepSpectra® samples, specific gravity was used as an initial distinguishing test between samples. The increase in relative density between the sample groups (0.04) is a good indicator that additional compounds, specifically those with a higher molecular weight, were extracted through the DeepSpectra® process. Specific gravity can be viewed as a quick and efficient means of evaluating DeepSpectra® extraction.
As previously mentioned, a total of 49 volatile compounds were identified by GC/MS analysis. Of the prominent volatile compounds detected (≥ 0.5% in at least one sample), only isocembrol had a higher standard deviation between all three associated samples (i.e., EO sample A and DeepSpectra® sample A, etc.) (σ = 0.2 for sample group A, σ = 0.2 for sample group B, σ = 0.3 for sample group C). While volatile profiles were seemingly unchanged from the initial extraction (EO) and DeepSpectra® extraction, isocembrol may be the exception as values displayed a higher standard deviation for all three sample groups and could be, as a diterpene alcohol (C20H34O), considered a semi-volatile aromatic compound that is most efficiently extracted during the secondary extraction process. These data suggest that the DeepSpectra® process minimally impacts the volatile compound profile of frankincense EO and that liquid chromatography is critical for identifying additional compounds extracted from the spent frankincense resin, specifically non-volatile compounds.
To support the specific gravity data between each sample set, non-volatile compound profiles were differentiated between frankincense EO and DeepSpectra® samples (Fig. 6).
Figure 6. Volatile (a-c) and non-volatile (d-f) compounds present in the samples. All essential oil and DeepSpectra® (DS) samples contained (a) α-pinene, (b) limonene, (c) α-phellandrene dimer; while (d) α-boswellic acid, (e) 11-keto-β-boswellic acid and (f) 3-acetyl-11-keto-β-boswellic acid were only detected in DS samples. Illustrated by Rick Simonson, Science Lab Studios, Inc. (Kearney, NE, USA).
While none of the non-volatile triterpenoids were detected in the frankincense EO samples, six were detected and quantified in each DeepSpectra® sample (α-boswellic acid, β-boswellic acid, 3-acetyl-11-keto-β-boswellic acid, 11-keto-β-boswellic acid, acetyl-α-boswellic acid and acetyl-β-boswellic acid). Of these six compounds, four (α-boswellic acid, β-boswellic acid, 3-acetyl-11-keto-β-boswellic acid and 11-keto-β-boswellic acid) were specifically determined to be highly bioactive in previousl studies [8,10,11,13]. Given the enhanced non-volatile compound profile of the DeepSpectra® samples, we may conclude that the extraction process investigated in this study does extract additional beneficial compounds from plant materials. Future studies should investigate the bioactivity and utility of DeepSpectra® samples in relation to the initially extracted essential oils.
With the additional LC/QTOF analysis, a scatter plot (Fig. 4) was created to visualize the relationship between the sample groups. Statistically significant (p < 0.05) differences were observed between the non-volatile profiles of frankincense EO and DeepSpectra® samples. While LC/QTOF is a reliable analytical technique for determining the exact mass of compounds, the identification of additional non-volatile compounds other than the six previously mentioned was not conducted and will be the focus of future research. However, the scatter plot suggests that with additional investigation and resources, many more non-volatile compounds could be confidently identified in the DeepSpectra® samples, further distinguishing the sample types and substantiating the novel extraction technique used herein.
4. Conclusions
Given that tree resins are comprised of both volatile and non-volatile fractions, distillates of resins, given their typical distillation properties, are prone to being composed only of volatile compounds. The current patents pending secondary extraction technique recovers non-volatile compounds that are otherwise not detected in resin essential oils.
Specific gravity was a reliable initial test to distinguish between Boswellia sacra (frankincense) essential oils and DeepSpectra® samples. GC/MS demonstrated that the volatile profiles of each sample type were similar. Differences in the non-volatile profiles between sample types were determined using LC/MS/MS analysis. Six boswellic acids (α-boswellic acid, β-boswellic acid, 3-acetyl-11-keto-β-boswellic acid, 11-keto-β-boswellic acid, acetyl-α-boswellic acid, and acetyl-β-boswellic acid) were detected in DeepSpectra® samples, but were not detected in any frankincense essential oil samples. Additionally, LC/QTOF demonstrated that the identification of other non-volatile compounds is feasible with additional research.
While the current study focuses on using the DeepSpectra® process on frankincense resin, it is fully reasonable to assume that the same extraction technique will work with a myriad of other plant materials. Future research will focus on using these patents pending extraction techniques for other plant species.
Abbreviations
The following abbreviations are used in this manuscript: EO (Essential Oil); DCM (Dichloromethane); DS (DeepSpectra®); GC/MS (Gas Chromatography/Mass Spectrometry); LC/MS/MS (Liquid Chromatography/Tandem Mass Spectrometry); LC/QTOF (Liquid Chromatography/Quadrupole Time-of-Flight); YLEO (Young Living Essential Oils).
Patents
United States Patent Application Publication Number: US 2024/0084217 A1. Publication Date: 14 March 2024. Publication Title: METHODS AND SYSTEMS FOR EXTRACTING ADDITIONAL BENEFICIAL LIPID-SOLUBLE COMPOUNDS FROM PLANT MATERIALS IN ENVIRONEMENTIALLY SUSTAINABLE WAYS.
Supplementary materials
Table S1. Complete gas chromatography dataset.
Supplementary material to this article can be found online at https://www.currentsci.com/images/articlesFile/supplementary.1751297439.pdf
Authors’ contributions
Conceptualization, T.M.W., H.K.L., C.P.; sample procurement and production, T.M.W.; methodology, T.M.W., T.T.M.; software, T.M.W., T.T.M.; validation, C.R.B.; formal analysis, T.M.W., T.T.M.; data curation, T.M.W., T.T.M.; writing—original draft preparation, T.M.W., T.T.M.; writing—review and editing, H.K.L, C.P., C.R.B.; funding acquisition, C.R.B.
Acknowledgements
The authors wish to thank the following individuals for their contributions: Andy Evans (YLEO), John Whetten (YLEO), and Rick Simonson (Science Lab Studios, Inc.). Additionally, the authors would like to thank Agilent Technologies (Santa Clara, CA, USA) for their collaboration on this project.
Funding
This research was funded by Young Living Essential Oils.
Availability of data and materials
All data are either presented within the current manuscript or accessible through the supplementary table (Table S1).
Conflicts of interest
The authors declare no conflict of interest. While the funders (Young Living Essential Oils) hold the patent for DeepSpectra® technology, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
1. | The World Flora Online. Available online: https://www.worldfloraonline.org/taxon/wfo-0000569724 (accessed on 19 March 2025). |
2. | Guenther, E. The Essential Oils, Vol IV.; Robert E. Krieger Publishing Co., Inc.: Huntington, NY, USA, 1950. |
3. | Al-Harrasi, A.; Al-Saidi, S. Phytochemical analysis of the essential oil from botanically certified oleogum resin of Boswellia sacra (Omani Luban). Molecules 2008, 13, 2181-2189. https://doi.org/10.3390/molecules13092181 |
4. | Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 686-691. https://doi.org/10.1016/j.sajb.2010.06.001 |
5. | Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Abdo Hasson, S.S.A.; Jabri A.A.; McCarron P.A.; Tambuwala M.M. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Oncotarget. 2019, 10, 3472-3490. https://doi.org/10.18632/oncotarget.26930 |
6. | Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. https://doi.org/10.1186/1472-6882-11-129 |
7. | Ni, X.; Suhail, M.M.; Yang, Q.; Cao, A.; Fung, K.M.; Postier, R.G.; Woolley, C.; Young, G.; Zhang, J.; Lin, H.K. Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement. Altern. Med. 2012, 12, 253. https://doi.org/10.1186/1472-6882-12-253 |
8. | Greve, H.L.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Boswellic acids show in vitro activity against Leishmania donovani. Molecules. 2021, 26, 3651. https://doi.org/10.3390/molecules26123651 |
9. | Jamshidi-Adegani, F.; Ghaemi, S.; Al-Hashmi, S.; Vakilian, S.; Al-Kindi, J.; Rehman, N.U.; Alam, K.; Al-Riyami, K.; Csuk, R.; Arefian, E.; Al-Harrasi, A. Comparative study of the cytotoxicity, apoptotic, and epigenetic effects of Boswellic acid derivatives on breast cancer. Sci. Rep. 2022, 12, 19979. https://doi.org/10.1038/s41598-022-24229-y |
10. | Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Tarahi, M.; Khan, T.S.; Alam, T.; Koca, E.; Ayemir, L.Y. Comparative study of pectin and kappa‐carrageenan‐based films loaded with boswellic acid and acetyl‐11‐keto‐beta‐boswellic acid for active packaging. J. Food Qual. 2025, 1, 3191264. https://doi.org/10.1155/jfq/3191264 |
11. | Khan, A.; Khan, I.; Halim, S.A.; Rehman, N.U.; Karim, N.; Ahmad, W.; Khan, M.; Csuk, R.; Al-Harrasi, A. Anti-diabetic potential of β-boswellic acid and 11-keto-β-boswellic acid: Mechanistic insights from computational and biochemical approaches. Biomed. Pharmacother. 2022, 147, 112669. https://doi.org/10.1016/j.biopha.2022.112669 |
12. | Javdani, M.; Dastjerdi, M.A.; Shirian, S. Effect of Boswellia serrata extract on tissue inflammation and white blood cells responses of spinal cord injury in rat model. J. Herbmed. Pharmacol. 2018, 7, 4, 273-279. |
13. | Sameni, S.; Khalsara, M.G.; Sameni, H.R. Acetyl-11-keto-beta-boswellic acid (AKBA) ameliorates histopathological and oxidative stress brain disorders in the experimental model of cuprizone-induced demyelination. J. Microbiota. 2024, 1, 3, e153658. https://doi.org/10.5812/jmb-153658 |
14. | Wadhwa, S.; Antal, S.; Kumari, R.; Kumar, D.; Sardana, I.; Tagra, V.K.; Anuradha. Evaluation of anti-depressant potential of boswellic acid in mice. Eur. Chem. Bull. 2023, 12, 4, 6484-505. |
15. | Lin, H.K. Methods and systems for extracting additional beneficial lipid-soluble compounds from plant materials in environmentally sustainable ways. US Patent 2024/0084217 A1, filed 8 September 2022, and issued 14 March 2024. |
16. | ISO 279:1998, Essential oils – Determination of relative density at 20 °C – Reference method. ISO 279. Available online: https://www.iso.org/standard/25308.html (accessed on 19 March 2025). |
17. | Adams, R.P.; Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th Edn.; Carol Stream, Allured Publ.: Illinois, USA, 2007. |
18. | Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, Ethnobotany; Timber Press, Inc.: Oregon, USA, 2003. |
19. | Wilson, T.M.; Ziebarth, E.A.; Carlson, R.E. Essential oil from naturally exuded Pseudotsuga menziesii var. glauca (Pinaceae) resin. J. Essent. Oil Plant Comp. 2023, 1, 3, 213-219. https://doi.org/10.58985/jeopc.2023.v01i03.27 |
20. | Jackson, R.B.; Wilson, T.M.; Packer, C.; Bowerbank, C.R.; Carlson, R.E. Essential oil from naturally exuded Pinus contorta var. latifolia (Pinaceae) resin. J. Essent. Oil Plant Comp. 2025, 3, 1, 37-44. https://doi.org/10.58985/jeopc.2025.v03i01.64 |
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
Essential oils (EO), sometimes called volatile oils, are produced by aromatic plants and extracted from various plant parts. EO have historically been extracted using various solvents, with water likely the most common solvent. EO are largely composed of volatile compounds, such as phenylpropanoids, monoterpenoids, sesquiterpenoids, and to a lesser extent, diterpenoids. The current study investigates a novel, patents pending extraction technique that uses EO as a natural solvent to extract additional, non-volatile compounds from plant materials that were otherwise devoid from EO. While the application is seemingly endless with various plant materials, the current study focuses on (1) using frankincense (Boswellia sacra) resin to produce an EO through hydrodistillation and then (2) using said EO as a solvent on the ‘spent’ frankincense resin to extract additional, non-volatile compounds. From the initial extraction, frankincense EO samples were largely comprised (determined by GC/MS) of α-pinene (avg. 39.8%), limonene (avg. 16.4%), and contained detectable compounds as large as α-phellandrene dimer (C20H32), albeit in trace amounts. From the secondary extraction, where EO was used as a solvent on spent frankincense resin, the volatile profile was similar, but additional non-volatile compounds were detected (determined by LC/MS/MS) such as α-boswellic acid (C30H48O3) (avg. 178.92 μg/mL), 3-acetyl-11-keto-β-boswellic acid (C32H48O5) (avg. 40.40 μg/mL), and 11-keto-β-boswellic acid (C30H46O4) (avg. 13.87 μg/mL). The current study establishes a novel and sustainable extraction technique that has potential applications across multiple industries, including the flavor and fragrance, and pharmaceutical industries.
Abstract Keywords
Boswellia sacra, boswellic acids, frankincense, monoterpenoids; resin, triterpenoids.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


