Research Article
Davies-Sani Rayhana Olubukola
Davies-Sani Rayhana Olubukola
Department of Chemistry, Lagos State University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
Elesho Adeseye Omololu
Elesho Adeseye Omololu
Department of Chemistry, Lagos State University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
Balogun Sunmisola Rukayat
Balogun Sunmisola Rukayat
Department of Chemistry, Lagos State University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
Azeez Ridwan Ajibola
Azeez Ridwan Ajibola
Department of Chemistry, Lagos State University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
Moses Sunday Owolabi*
Moses Sunday Owolabi*
Corresponding
Author
Department of Chemistry, Lagos State
University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
E-mail: moses.owolabi@lasu.edu.ng, sunnyconcept2007@yahoo.com,
Tel: +2348033257445
Lanre Akintayo Ogundajo
Lanre Akintayo Ogundajo
Department of Chemistry, Lagos State University, Ojo, P.M.B 001, LASU, Lagos, Nigeria.
Prabodh Satyal
Prabodh Satyal
Aromatic Plant Research Center, 230 N 1200 E, Suite 100, Lehi, UT 84043, USA.
Ambika Poudel
Ambika Poudel
Aromatic Plant Research Center, 230 N 1200 E, Suite 100, Lehi, UT 84043, USA.
William N. Setzer
William N. Setzer
Aromatic Plant Research Center, 230 N
1200 E, Suite 100, Lehi, UT 84043, USA
And
Department of Chemistry, University of
Alabama in Huntsville, Huntsville, AL 35899, USA
E mail: setzerw@uah.edu, wsetzer@chemistry.uah.edu;
Tel.: +1-256-468-2862
Received: 2025-08-09 | Revised:2025-08-19 | Accepted: 2025-08-20 | Published: 2025-08-23
Pages: 133-139
DOI: https://doi.org/10.58985/jeopc.2025.v03i02.72
Abstract
The genus Croton is important in
local traditional medicines wherever these species are found. In addition, Croton
essential oils have shown notable biological activities, which can be
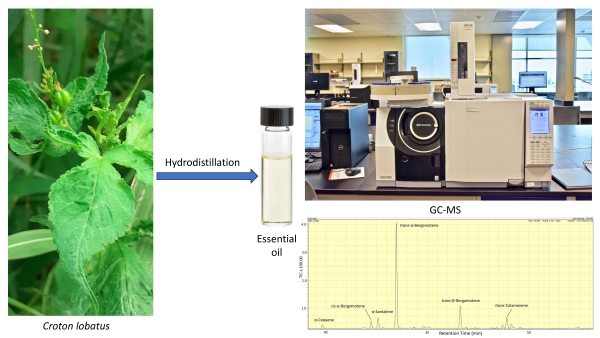
attributed to their major components. In this study, the leaf essential oil of Croton
lobatus from north-central Nigeria was obtained by hydrodistillation (0.45%
w/w yield) and analyzed by gas chromatography – mass spectrometry. The
essential oil was dominated by sesquiterpene hydrocarbons (93.0%), including trans-α-bergamotene
(56.9%), trans-β-bergamotene (12.9%), trans-calamenene (6.4%),
and α-santalene (6.0%). A literature survey revealed that several Croton
leaf essential oils are also rich in sesquiterpene hydrocarbons. However, (E)-β-caryophyllene
and bicyclogermacrene were the most common. So far as we know, this is the
first report on the essential oil composition of C. lobatus and serves
to add to our understanding of the phytochemistry of this important genus.
Keywords
Euphorbiaceae, bergamotene, calamenene,
gas chromatography, mass spectrometry.
1. Introduction
Croton (Euphorbiaceae) is one of the largest
aromatic genera of flowering plants, comprising of about 1300 species. The
plant is native to South America, Asia, and West Africa, but it is distributed
among all the tropics and subtropics of the world with high ecological
importance [1-3]. Croton species have
been used to treat a wide range of illnesses, infections, and digestive issues [4-6], as well as sources of food flavoring and
spices [7-9].
Croton
lobatus L. (syn. Astraea lobata (L.) Klotzsch) is a perennial
woody herbaceous shrub plant with common medicinal and ecological importance
widely spread in Caribbean countries as well as tropical Africa [10]. C. lobatus is known as
lobed croton in English [11], while in
Nigeria, the plant is called Gaásàyaá (Hausa), Òkwè (Igbo), Àjẹìofòlé or ẹÌru (Yoruba) [3]. Ethnobotanically, decoctions of C. lobatus are used in folk medicine for
the treatment of several ailments, for example malaria, dysentery, pregnancy
troubles, hypertension, ulcers, diabetes, and other diseases [9]. Previous studies have revealed that C. lobatus contains significant amounts
of primary metabolites, such as protein, carbohydrates, and fibers, which serve
as food supplements to remedy malnutrition in traditional medicine [12, 13]. Compounds isolated
from Croton species have been reported to exhibit a range of biological properties, including
cytotoxic, anti-inflammatory, antifungal, acetylcholinesterase inhibitory, and
neurite outgrowth-promoting activities [5, 9, 14-16].
The non-volatile phytochemicals from C. lobatus have been
reviewed [17]. The isolated and identified compounds
included the triglyceride lobaricide, triterpenoid betulinic acid, alkaloids,
tannins, and saponins. The
plant is believed to be toxic, but it is an important livestock feed in Namibia
[9]. Its essential oils and phytochemicals
were investigated and found to have antibacterial properties, but little
antioxidant properties due to the minimal concentrations of polyphenols in the
investigated extracts [10, 12, 18, 19]. As part of our interest in the chemical
characterization of essential oils of aromatic and medicinal plants of the Euphorbiaceae family, we present the essential oil
composition of C. lobatus leaves
collected from north-central Nigeria. To the best of our knowledge, there have
been no previous reports on the volatile components of C. lobatus.
2.
Materials and methods
2.1. Plant material collection and
identification
Fresh leaves
of Croton lobatus (1.5 kg) were
collected in February 2024 from Saboganri (11.1766° N, 7.6765° E) in the Saboganri
local government, Kaduna state, Nigeria. The plant was validated by Mr. Namadi
Sunusu of the Botany Department, Ahmadu Bello University, Zaria, where a
voucher specimen (08145) was deposited at the herbarium. The leaves of C. lobatus were air dried under shade
for 5-7 days and pulverized using an electric blender and stored in a polythene
container until ready for use.
2.2. Sample preparation for essential oil
The air-dried leaf sample (500 g) was
placed in a 5-L flask, and distilled water was added to cover the sample.
Hydrodistillation was carried out for four hours in an all-glass Clevenger
apparatus in accordance with the British Pharmacopoeia. The distillate was
extracted with n-hexane, transferred to a pre-weighed amber sample vial,
and dried with anhydrous sodium sulfate to remove any remaining water to give a
light yellow essential oil. The oils were refrigerated at 4 °C until ready for analysis.
2.3. Gas
chromatographic – mass spectral analysis
The
chemical composition of the leaf essential oil from C. lobatus was
determined using gas chromatography–mass spectrometry (GC–MS). This was achieved
using a Shimadzu GCMS-QP2010 Ultra operated in electron impact (EI) mode
(electron energy = 70 eV), scan range = 40–400 atomic mass units, with a scan
rate of 3.0 scans per s, with GC–MS solution software. The GC column was a ZB-5
fused silica capillary column (30 m length × 0.25 mm inner diameter) with a 5%
phenyl-polymethylsiloxane stationary phase and a film thickness of 0.25 μm.
Helium gas was used as the carrier gas with a column head pressure of 552 kPa
at a flow rate of 1.37 mL/min. The injector and ion source temperatures were 250
°C and 200 °C, respectively. The oven temperature of 50 °C was initially
programmed for the GC and gradually increased at 2 °C/min to 260 °C. The sample
(5% w/v) was dissolved in dichloromethane, and 0.1 μL of the solution was
injected using a split-injection technique (30:1). Each constituent of the
essential oils was identified by injection of pure samples and by comparison of
the retention index values, through calibration using a series of n-alkanes
[20], in addition to MS fragmentation
comparisons with those found in databases [21-24].
3.
Results and discussion
3.1.
Essential oil profiles
The essential oil from the leaves of C. lobatus was obtained as a pale-yellow oil with a percentage yield of 0.45% (v/w) calculated on the basis of the weight of air-dried plant material to the volume of EO obtained. The total oil composition revealed 17 chemical constituents with a percentage composition of 94.8% (Table 1).
Table 1. Chemical composition of the leaf essential oil of Croton lobatus L. (Euphorbiaceae).
|
RT |
RIcalc |
RIdb |
Compounds |
Composition
(%) |
|
39.83 |
1377 |
1375 |
α-Copaene |
2.3 |
|
42.23 |
1414 |
1416 |
cis-α-Bergamotene |
4.0 |
|
42.58 |
1419 |
1418 |
α-Santalene |
6.0 |
|
42.82 |
1423 |
1421 |
(E)-α-Ionone |
0.4 |
|
43.47 |
1434 |
1432 |
trans-α-Bergamotene |
56.9 |
|
44.35 |
1448 |
1446 |
epi-β-Santalene |
0.6 |
|
44.83 |
1456 |
1454 |
α-Humulene |
1.1 |
|
45.10 |
1460 |
1457 |
β-Santalene |
0.7 |
|
46.62 |
1484 |
1483 |
trans-β-Bergamotene |
12.9 |
|
46.91 |
1489 |
1492 |
β-Selinene |
0.6 |
|
47.36 |
1496 |
1497 |
α-Selinene |
0.6 |
|
48.17 |
1509 |
1508 |
β-Bisabolene |
0.9 |
|
48.42 |
1514 |
1514 |
Sesquicineole |
0.7 |
|
48.72 |
1519 |
1518 |
δ-Cadinene |
2.0 |
|
48.91 |
1522 |
1519 |
trans-Calamenene |
6.4 |
|
49.57 |
1533 |
1536 |
trans-Cadina-1,4-diene |
0.8 |
|
52.44 |
1582 |
1587 |
Caryophyllene
oxide |
0.7 |
|
|
|
|
Compound
classes |
|
|
|
|
|
Sesquiterpene
hydrocarbons |
93.0 |
|
|
|
|
Oxygenated
sesquiterpenoids |
1.4 |
|
|
|
|
Others |
0.4 |
|
|
|
|
Total
identified |
94.8 |
RT = Retention time (min). RIcalc = Retention index determined with respect to a homologous series of n-alkanes on a ZB-5ms column [20]. RIdb = Reference retention index values from the databases [21-24].
C. lobatus essential oil was dominated by sesquiterpene hydrocarbons (93.0%), predominantly trans-α-bergamotene (56.9%), trans-β-bergamotene (12.9%), trans-calamenene (6.4%), α-santalene (6.0%), cis-α-bergamotene (4.0%), α-copaene (2.3%) and δ-cadinene (2.0%), with trace amount of oxygenated sesquiterpenoids. These findings differ from the essential oil compositions of other Croton species (Table 2). The chemical profiles of other Croton essential oils, when compared with our sample, were also dominated by varying amounts and types of sesquiterpene hydrocarbons, as shown in Table 2.
Table 2. Comparison of the major ( > 5 %) sesquiterpene hydrocarbons in the leaf essential oils of Croton species.
Croton species | Collection site | Major sesquiterpene hydrocarbon components | Ref. |
C. argyrophylloides Müll. Arg. | Fortaleza, CE, Brazil | trans-b-Elemene (7.9%) | [25] |
C. argyrophylloides Müll. Arg. | Viçosa do Ceará, CE, Brazil | Bicyclogermacrene (30.6%), trans-β-elemene (8.52%), | [26] |
C. argyrophyllus Kunth | Alagoas, Maceió, AL, Brazil | Bicyclogermacrene (27.8%), δ-elemene (8.7%), trans-β-elemene (8.5%), prenopsan-8-ol (8.5%), (E)-β-caryophyllene (6.3%), trans-dauca-4(11),7-diene (6.0%) | [27] |
C. bonplandianus Baill. | Belgaum, Karnataka, India | (E)-β-Caryophyllene (16.7%), germacrene D (14.7%), (Z)-β-damascenone (6.9%), α-humulene (6.1%), germacrene A (5.2%) | [28] |
C. campestris A. St.-Hil. | Crato, CE, Brazil | (E)-β-Caryophyllene (17.0%), bicyclogermacrene (16.2%) | [29] |
C. campestris A. St.-Hil. | Belém, PA, Brazil | (E)-β-Caryophyllene (23.0%), γ-elemene (13.9%), germacrene D (13.7%), trans-β-elemene (7.1 %), δ-elemene (6.0%), bicyclogermacrene (4.7%) | [30] |
C. campestris A. St.-Hil. | São Paulo, Brazil | (E)-β-Caryophyllene (43.0%), α-cubebene (15.8%) | [31] |
C. chaetocalyx Müll. Arg. | Belém, PA, Brazil | Bicyclogermacrene (13.9%), δ-elemene (13.5%), germacrene D (9.3%), spathulenol (9.0%), δ-cadinene (8.0%), (E)-β-caryophyllene (7.1%) | [30] |
C. doctoris S. Moore | Dourados, MS, Brasil | (E)-β-caryophyllene (39.6%), α-humulene (13.2%) | [32] |
C. draconoides Müll. Arg. | Belém, PA, Brazil | Curzerene (12.8%), germacrene D (9.0%) | [33] |
C. eriocladus Müll. Arg | Belém, PA, Brazil | (E)-β-Caryophyllene (24.1%), germacrene D (17.9%), α-humulene (6.2%), bicyclogermacrene (5.2%) and δ-elemene (5.0%) | [30] |
C. flavens L. | Chicoutimi, Quebec, Canada | Viridiflorene (12.2%), (E)-γ-bisabolene (5.3%) and (E)-β-caryophyllene (5.0%) | [34] |
C. glandulosus Vell. | São Paulo, Brazil | (E)-β-Caryophyllene (18.9%) | [31] |
C. glandulosus Vell | Belém, PA, Brazil | Bicyclogermacrene (9.6%), (E)-β-caryophyllene (8.9%), δ-elemene (8.8%) | [30] |
C. heliotropiifolius Kunth | Caatinga biome, Pernambuco-Brazil | (E)-β-Caryophyllene (20.8%), spathulenol (16.4%), germacrene B (9.3%) | [35] |
C. heliotropiifolius Kunth | Semi-arid region, northeast Brazil | (E)-β-Caryophyllene (35.8%), bicyclogermacrene (20.0%), germacrene D (11.9%) | [36] |
C. heliotropiifolius Kunth | São Francisco, Petrolina, PE, Brazil | (E)-β-Caryophyllene (28.6-47.0%), bicyclogermacrene (22.6%), germacrene D (7.8%) | [37] |
C. heliotropiifolius Kunth | São Paulo, Brazil | (E)-β-Caryophyllene (23.9%), γ-muurolene (10.5%), viridiflorene (8.1%) | [38] |
C. jacobinensis Müll. Arg. | Fortaleza, CE, Brazil | Germacrene B (7.6%), | [25] |
C. jacobinensis Müll. Arg. | Viçosa do Ceará, CE, Brazil | Bicyclogermacrene (30.1%), β-elemene (22.3%), δ-elemene (12.9%), (E)-β-caryophyllene (10.9%), α-humulene (7.6%) | [26] |
C. jacobinensis Müll. Arg. | Caatinga biome, Pernambuco, Brazil | No major sesquiterpene hydrocarbons | [39] |
C. lobatus L. | Northwest Nigeria | trans-α-Bergamotene (56.9%), trans-β-bergamotene (12.9%), trans-calamenene (6.4%), α-santalene (6.0%) | This study |
C. lundianus Müll. Arg. | São Paulo, Brazil | γ-Elemene (19.0%), α-cubebene (17.1 %), (E)-β-caryophyllene (11.1%) | [31] |
C. matourensis Aubl. | Caracas, Venezuela | Valencene (5.8%) | [40] |
C. micans Sw. | Caatinga biome, Pernambuco, Brazil | α-Bulnesene (32.9%), germacrene B (21.4%), (E)-β-caryophyllene (12.3%) | [39] |
C. micans Sw. | Caracas, Venezuela | α-Humulene (12.6%), (E)-β-caryophyllene (5.5%), β-cubebene (5.0%), α-cubebene (5.3%) | [40] |
C. muscicapa Müll.Arg. | Caatinga biome, Pernambuco, Brazil | (E)-β-caryophyllene (7.0%) | [39] |
C. nepetifolius Baill. | Fortaleza, CE, Brazil | (E)-β-Caryophyllene (21.2%), germacrene B (5.8%) | [25] |
C. rhamnifolius Kunth | Caatinga biome, Pernambuco, Brazil | (E)-β-Caryophyllene (7.3%) | [39] |
C. sincorensis Mart. ex Müll. Arg. | Viçosa do Ceará, CE, Brazil | (E)-β-Caryophyllene (25.3%), bicyclogermacrene (23.9%), germacrene D (9.1%), trans-β-elemene (5.6%), | [26] |
C. sincorensis Mart. ex Müll. Arg. | Fortaleza, CE, Brazil | No major sesquiterpene hydrocarbons | [25] |
C. triqueter Lam. | Belém, PA, Brazil | (E)-β-caryophyllene (16.3%), β-phellandrene (10.2%), | [33] |
C. urucurana Baill. | Belém, PA, Brazil | Sesquicineole (23.0%), dehydro-sesquicineole (13.8%), (E)-β-caryophyllene (7.9%), | [33] |
C. zambesicus Muell. Arg. | Southwestern Nigeria: | (E)-β-Caryophyllene (8.8%) | [41] |
(E)-β-Caryophyllene and bicyclogermacrene were common in most Croton species reported, but these constituents were not found in our sample. In contrast, our sample predominantly contained trans-α-bergamotene and trans-β-bergamotene. To the best of our knowledge, this is the first report on the chemical profile of C. lobatus.
Moreover, studies have shown that bicyclogermacrene, a sesquiterpene essential oil constituent, that exhibited antibacterial, antioxidant, antispasmodic, antiviral, and analgesic activities [42-44]. In addition, the non-volatile phytochemical constituents of Croton species containing diterpenoids have been reported to treat various ailments and diseases [45].
4. Conclusions
This is the first report on the essential oil composition of Croton lobatus. The essential oil was dominated by sesquiterpene hydrocarbons, including trans-α-bergamotene (56.9%), trans-β-bergamotene (12.9%), trans-calamenene (6.4%), and α-santalene (6.0%). However, the sesquiterpene hydrocarbon composition was remarkably different from previous reports on the leaf essential oils of Croton species, which are often dominated by (E)-β-caryophyllene and bicyclogermacrene. Thus, this study complements previous investigations on the Croton essential oils and adds to our knowledge of the phytochemical characterization of this genus. The high concentration of trans-α-bergamotene may make C. lobatus an attractive source of this sesquiterpene.
Authors’ contributions
Conceptualization, M.S.O; Methodology, D.S.R.O., E.A.O., M.S.O., P.S., W.N.S.; Software, P.S.; Validation, A.L.O., W.N.S., Formal Analysis, A.P., W.N.S.; Investigation, D.S.R.O., E.A.O., B.S.R., M.S.O., P.S., A.P., W.N.S.; Resources, D.S.R.O., M.S.O. A.R.A.., P.S., W.N.S.; Data curation, W.N.S.; Writing – original draft preparation, D.S.R.O. B.S.A., A.R.A., M.S.O; Writing – review & editing, M.S.O., W.N.S.; Project administration, D.S.R.O., M.S.O.
Acknowledgements
This work was carried out as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Funding
This research received no specific grant from any funding agency.
Availability of data and materials
All data will be made available on request according to the journal policy.
Conflicts of interest
The authors declare no conflict of interest.
References
1. | Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A. A New Name and New Combinations in the Euphorbiaceae: Euphorbiinae Kew Bulletin. 1996. https://doi.org/10.2307/4117028 |
2. | Webster, G.L. A Provisional synopsis of the sections of the genus Croton (Euphorbiaceae). Taxon. 1993, 42(4), 793-823. https://doi.org/10.2307/1223265 |
3. | Burkil, H.M. The useful plants of west tropical Africa, Vol. 1, Second Ed., Royal Botanic gardens; Kew, UK, 2000, p. 976. ISBN: 978-0439836814 |
4. | Wu, X.; Zhao, Y. Advance on chemical composition and pharmacological action of Croton L Natural product research and development. 2004, 16(5), 467-472. https://api.semanticscholar.org/CorpusID:87238288 |
5. | Odukoya, O.A.; Sofidiya, M.O.; Samuel, A.T.; Ajose, I.; Onalo, M.; Shuaib, B. Ethnobotnicalstudy of wound healing plants in Lagos-Nigeria. Planta Medica. 2009, 75-PJ5. https://doi.org/10.1055/s-0029-1234810 |
6. | Xu, W.H.; Liu, W.Y.; Liang, Q. Chemical constituents from Croton species and their biological activities. Molecules. 2018, 23(9), 2333. https://doi.org/10.3390/molecules23092333 |
7. | Mahmoud, A.B.; Danton, O.; Kaiser, M.; Khalid, S.; Hamburger, M.; Mäser, P. HPLC-based activity profiling for antiprotozoal compounds in Croton gratissimus and Cuscuta hyalina. Front. Pharmacol. 2020, 11(55), 599. https://doi.org/10.3389/fphar.2020.01246 |
8. | Adamu, T.; Atanda, H.A.; Al-Adams, B.K.; Nwaokoro, S.C.; Isyaka, M.S; Abdullahi, M.A. Proximate analysis of Nigerian Croton gratissimus, Croton lobatus, Croton membranaceus and Croton penduliflorus. Fudma J. Sci. 2014, 8(3), 204-207. https://doi.org/10.33003/fjs-2024-0803-2547 |
9. | Weniger, B.; Lagnika, L.; Vonthron-Sénécheau, C.; Adjobimey, T.; Gbenou, J.; Moudachirou, M.; Sanni, A. Evaluation of ethnobotanically selected Benin medicinal plants for their in-vitro antiplasmodial activity. J. Ethnopharmacol. 2004, 90(2-3), 279-284. https://doi.org/10.1016/j.jep.2003.10.002 |
10. | Block, S.; Baccelli, C.; Tinant, B.; Meervelt, L.C.; Rozenberg, R.; Jiwan, J.L.H.; Llabrès, G.; Pauw-Gillet, M.C.; Quetin-Leclercq, J. Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin activity. Phytochem. 2004, 65, 1165. https://doi.org/10.3390/molecules191016573 |
11. | eFloras.org. Astraea lobata (Linnaeus) Klotzsch. Flora of North America, Vol. 12. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=250057734 |
12. | Isyaka, M.S.; Al-Adam, K.B.; Umar, A.; Adedoyin, B.; Mofio, B.M.; Bemgba, B.N.; Abdu, Z.; Jibril, S.; Rufai, Y.; Anyam, J.V. Phytochemistry and bioactivity of Nigerian Croton lobatus L. stem bark. Trop. J. Phytochem. Pharm. Sci. 2025, 4, 79-84. https://doi.org/10.26538/tjpps/v4i2.8 |
13. | Yibralign, Z. Phytochemical investigation on the stem bark of Croton macrostachyuss (Bisana). Master’s Thesis. Addis Ababa University, Addis Ababa. 2007. |
14. | Kishor, B.; Ghosh, C. Astraea lobata (L.) Klotzsch [Euphorbiaceae]: A new record for eastern India from Darjeeling Terai Pleione. 2019, 13(2), 422-424. https://doi.org/10.26679/pleione.13.2.2019.422-424 |
15. | Lane, M.A.; Edwards, J.L. Global biodiversity information facility, GBIF. Backbone Taxonom. 2016, 20, 17. https://doi.org/10.1201/9781439832547-1 |
16. | Malpighiales of North America Update, database; Retrieved from http://www.itis.gov/servlet/ SingleRpt/SingleRpt? 2011. |
17. | Isyaka, S.M.; Akintayo, A.H.; Abdullahi, A.M. A review of ethnomedicinal uses, phytochemistry and pharmacology of Nigerian Crotons. Arch. Curr. Res. Int. 2024, 24, 250-274. https://doi.org/10.9734/acri/2024/v24i121016 |
18. | Xu, W.H..; Liu, W.L.; Liang, Q. Chemical constituents from Croton species and their biological activities. Molecules. 2018, 23, 2333. https://doi.org/10.3390/molecules23092333 |
19. | Chodaton-Zinsou, M.D.; Assogba, F.A.; Yayi-Ladékan, E.; Gbaguidi, F.; Moudachirou, M.; Gbénou, J.D. Phytochemical composition, biological activities of Croton lobatus L. leaves, hydrolysis effect on activities and chemical composition. Am. J. Appl. Chem. 2020, 8(1), 13-22. https://doi.org/10.11648/j.ajac.20200801.13 |
20. | van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatog. A. 1963, 11, 463–471. https://doi.org/10.1016/s0021-9673(01)80947-x |
21. | Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; 4th ed.; Allured Publishing: Carol Stream, Illinois, USA, 2007, ISBN 978-1-932633-21-4. |
22. | Mondello, L. FFNSC 3. Shimadzu Scientific Instruments: Columbia, Maryland, USA, 2016. |
23. | NIST20. National Institute of Standards and Technology: Gaithersburg, Maryland, USA, 2020. |
24. | Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils, Ph.D. dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. |
25. | Morais, S.M.; Cossolosso, D.S.; Silva, A.A.S.; Filho M.O.M.; Teixera, J.M.; Campello, C.C.; Bonilla, O.H.; de Paula Júnior, V.F.; Vila-Nova, N.S. Essential oils from Croton species: Chemical composition, in vitro and in silico antileishmanial evaluation, antioxidant and cytotoxicity activities. J. Braz. Chem. Soc. 2019, 30(1), 2404-2412. http://dx.doi.org/10.21577/0103-5053.20190155 |
26. | de Souza, G.S.; Bonilla, O.H.; de Lucena, E.M.P; Barbosa, Y.P. Chemical composition and yield of essential oil from three Croton species. Ciência Rural. 2017, 47(8), e20161054. https://doi.org/10.1590/0103-8478cr20161054 |
27. | Araujo, S.S.; Santos, M.I.S.; Dias, A.S.; Ferro, J.N.S.; Lima, R.N.; Barreto, E.O.; Corrêa, C.B.; Araújo, B.S.; Lauton-Santos, S.; Shan, A.Y.K.; Alves, P.B.; Santana, A.E.G.; Thomazzi, S.M.; Antoniolli, A.R.; Estevam, C.S. Chemical composition and cytotoxicity analysis of the essential oil from leaves of Croton argyrophyllus Kunth. J. Essent. Oil Res. 2014, 1-6. http://dx.doi.org/10.1080/10412905.2014.956233 |
28. | Joshi, R.K. Chemical composition of the essential oil of Croton bonplandianus from India, Nat. Prod. Commun. 2014, 9(2), 269-270. https://doi.org/10.1177/1934578x1400900234 |
29. | de Almeida, T.S.; Rocha, J.B.T.; Rodrigues, F.F.G.; Campos, A.R.; da Costa, J.G.M. Chemical composition, antibacterial and antibiotic modulatory effect of Croton campestris essential oils. Ind. Crops Prod. 2013, 44, 630–633 http://dx.doi.org/10.1016/j.indcrop.2012.09.010 |
30. | Turiel, N.A.; Ribeiro, A.F.; Carvalho, N.C.C.; Monteiro, O.; Lucas, F.; Carreira, L.; Andrade, E.; Maia, J.G.S. Variability in essential oil composition of Croton species with occurrence in the eastern Amazon. Rec. Nat. Prod. 2016, 10(3), 380-384. https://www.researchgate.net/publication/283558658. |
31. | Compagnone, R.S.; Chavez, K.; Mateu, E.; Orsini, G.; Arvelo, F.; Suárez, A.I. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Rec. Nat. Prod. 2010, 4(22), 101-108. https://www.acgpubs.org/RNP © Published 04/ 25/2010 EISSN:1307-6167 |
32. | Cândido, A.C.S.; Scalon, S.P.Q.; Silva, C.B.; Simionatto, E.; Morel, A.F.; Stüker, C.Z.; Matos, M.F.C.; Peres, M.T.L P. Chemical composition and phytotoxicity of essential oils of Croton doctoris S. Moore (Euphorbiaceae) Braz. J. Biol. 2022, 82, e231957. https://doi.org/10.1590/1519-6984.231957 |
33. | Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. https://doi.org/10.1016/j.jep.2005.07.011 |
34. | Neves, I.A.; Camara, C.A.G. Volatile constituents of two Croton species from Caatinga biome of Pernambuco-Brasil. Rec. Nat. Prod. 2012, 6, 161–165. www.acgpubs.org/RNP © Published 10/23/2011 EISSN: 1307-6167 |
35. | Angelico, E.C.; Rodrigues, O.G.; Costa, J.G.M.; Lucena, M.F.A.; Neto, V.Q.; Medeiros, R.S. Chemical characterization and antimicrobial activity of essential oils and Croton's varieties modulator in the Brazilian's Northeast semiarid. Afr. J. Plant Sci. 2014, 8, 392–397.https://doi.org/10.5897/ajps2014.1198 |
36. | Filho, J.M.T.A.; Araújo, L.C.; Olivera, A.P.; Guimarães, A.L.; Pacheco, A.G.M.; Silva, F.S.; Cavalcanti, L.S.; Lucchese, A.M.; Almeida, J.R.G.S.; Araújo, E.C.C. Chemical composition and antibacterial activity of essential oil from leaves of Croton heliotropiifolius in different seasons of the year. Revista Bras. Farmacog. 2017, 27, 440–444 http://dx.doi.org/10.1016/j.bjp.2017.02.004 |
37. | Araújo, F.M.; Dantas, M.C.S.M.; e Silva, L.S.; Aonab, L.Y.S.; Tavares, I.F.; de Souza-Neta, L.C. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia, Brazil, Ind. Crop. Prod. 2017, 105, 203–206. https://doi.org/10.1016/j.indcrop.2017.05.016 |
38. | Ilzenayde, A.N.; da Camara, C.A.G. Acaricidal activity against Tetranychus urticae and essential oil composition of four Croton species from Caatinga biome in northeastern Brazil. Nat. Prod. Commun. 2011, 6(6), 893-899. https://doi.org/10.1177/1934578x1100600633 |
39. | Ogasawara, C.D. Chemical constituents and antioxidant and antiproliferative activities of Astraea Klotzsch and Croton L. (Euphorbiaceae) extracts. M.S. Thesis, University of São Paulo. 2012. |
40. | Turiel, N.A.; Ribeiro, A.F.; Carvalho, E.E.N.; Domingos, V.D.; Lucas, F.C.A.; Carreira, L.M.M.; Andrade, E.H.A.; Maia, J.G.S. Essential oils composition of Croton species from the Amazon. Nat. Prod. Commun. 2013, 8(10), 1471–1472. https://doi.org/10.1177/1934578x1300801034 |
41. | Owolabi, S.M.; Lawal, A.O.; Labunmi, L.; Hauser, R.M.; Setzer, W.N. Leaf oil composition of Croton zambesicus Muell. Arg. growing in southwestern Nigeria: Essential oil chemotypes of C. zambesicus. Am. J. Essent. Oils Nat. Prod. 2013, 1(1), 14-17. |
42. | Shafaghat, A. Antibacterial activity and GC/MS analysis of the essential oils from flower, leaf and stem of Origanum vulgare ssp. viride growing wild in northwest Iran. Nat. Prod. Commun. 2011, 6(9), 1351–1352. https://doi.org/10.1177/1934578x1100600933 |
43. | Costa, E.V.; Dutra, L.M.; de Jesus, H.C.R.; Nogueira, P.C.L.; Moraes, V.R.S.; Salvador, M.J.; Cavalcanti, S.C.H.; dos Santos, R.L.C.; Prata, A.P.N. Chemical composition and antioxidant, antimicrobial, and larvicidal activities of the essential oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Prod. Commun. 2011, 6 (6), 907–912. https://doi.org/10.1177/1934578x1100600636 |
44. | Horváth, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. b-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Rad. Biol. Med. 2012, 52, 1325–1333. https://doi.org/10.1016/j.freeradbiomed.2012.01.014 |
45. | Dória, G.A.A.; Silva, W.J.; Carvalho, G.A.; Alves, P.B.; Cavalcanti, S.C.H. A study of the larvicidal activity of two Croton species from northeastern Brazil against Aedes aegypti. Pharm. Biol. 2010, 48, 615–620. https://doi.org/10.3109/13880200903222952 |
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
The genus Croton is important in
local traditional medicines wherever these species are found. In addition, Croton
essential oils have shown notable biological activities, which can be
attributed to their major components. In this study, the leaf essential oil of Croton
lobatus from north-central Nigeria was obtained by hydrodistillation (0.45%
w/w yield) and analyzed by gas chromatography – mass spectrometry. The
essential oil was dominated by sesquiterpene hydrocarbons (93.0%), including trans-α-bergamotene
(56.9%), trans-β-bergamotene (12.9%), trans-calamenene (6.4%),
and α-santalene (6.0%). A literature survey revealed that several Croton
leaf essential oils are also rich in sesquiterpene hydrocarbons. However, (E)-β-caryophyllene
and bicyclogermacrene were the most common. So far as we know, this is the
first report on the essential oil composition of C. lobatus and serves
to add to our understanding of the phytochemistry of this important genus.
Abstract Keywords
Euphorbiaceae, bergamotene, calamenene,
gas chromatography, mass spectrometry.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


