Research Article
Njong Clementine Endam
Njong Clementine Endam
Department
of Nutrition, Food and Bioresource Technology, College of Technology,
University of Bamenda, P.O. Box 39, Cameroon.
E-mail: clemseybanks@yahoo.com, Tel: (+237) 679993344.
Feumba Dibanda Romelle*
Feumba Dibanda Romelle*
Corresponding
Author
Department
of Biochemistry and Molecular Biology, University of Buea, Cameroon,
E-mail: romellefeumba@gmail.com, Tel: (+237) 679993344.
Alex Dimitri Tchuenchieu Kamgain
Alex Dimitri Tchuenchieu Kamgain
Institute
of Medical Research and Medicinal Plants Studies- CRASAN, Yaoundé, Cameroon.
E-mail: tkad01@yahoo.fr
Okamba Elouti Renaud Blanchard
Okamba Elouti Renaud Blanchard
Department
of Microbiology, University of Yaounde 1, Cameroon.
E-mail: eloutiblanchard@yahoo.fr
Momo Kenfack Chancel Hector
Momo Kenfack Chancel Hector
Department
of Nutrition, Food and Bioresource Technology, University of Bamenda, Cameroon,
E-mail: momohector@yahoo.fr
Nwachan Mirabelle Boh
Nwachan Mirabelle Boh
Department
of Nutrition, Food and Bioresource Technology, University of Bamenda, Cameroon.
E-mail: nwachanmirabelle@gmail.com
Ejoh Richard Aba
Ejoh Richard Aba
Department
of Nutrition, Food and Bioresource Technology, University of Bamenda, Cameroon.
And
Department
of Food Science and Nutrition, National School of Agro-Industrial Sciences,
University of Ngoundere, Cameroon.
E-mail: ejohrab62@gmail.com
Abstract
This study aimed to identify lactic acid bacteria (LAB) from fresh African indigenous leafy vegetables (AILVs) consumed in Cameroon and screen them for total phenolic content and antioxidant capacity upon fermentation. The LAB were isolated from four AILVs; Solanum scabrum (African nightshade, AN), Telfeiria occidentalis (fluted pumpkin, FP), Amaranthus hybridus (Amaranthus vegetable, AL) and Talinum triangulaire (Waterleaf, WL). The LAB isolates were identified biochemically and molecularly. The LAB strains were then preserved in 20% glycerol at -20°C and later reactivated to carry out fermentation of the AILVs. Fermentation trials using a systematic replicate screening design, were conducted at 27°C with each LAB strain over a period of 0-4 days. The pH, total phenolics (FrP and BF), and antioxidant activities; ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl(DPPH) and total antioxidant capacity (TAC) were assessed using standard methods. The LAB isolates were identified by 16S rRNA gene sequencing as Lactiplantibacillus plantarumL52, Lactiplantibacillus plantarumL31, Leuconostoc mesenteroidesL33, Leuconostoc mesenteroidesL2 and Lactiplantibacillus pentosusL7. Fermentation with L. plantarumL52 strain significantly (p ≤ 0.05) decreased the pH from 6.39±0.006 to 3.82±0.006 in the AILVs. L. plantarumL31 fermented AILVs significantly increased (by 56.6% and 140.2%) FrP content and TAC activity respectively while L. mesenteroidesL33 significantly recorded the highest percentage DPPH (7.7%) and FRAP (106.9%) activities after 4 days. The most improved active metabolite trend was observed at 3 days of fermentation after screening. Overall, L. plantarumL31 and L. mesenteroidesL33 were the most functionally effective strains, and AN emerged as the most potent substrate among the vegetables. examined.
Keywords
Lactic acid bacteria, African indigenous leafy vegetables, fermentation, antioxidant activity, phenolic content.
1. Introduction
African indigenous leafy vegetables (AILVs) are native to sub-Saharan Africa (SSA) [1]. Their leaves, young shoots, flowers, fruits, seeds, stems, and roots are consumable and have been part of food systems for generations. These vegetables contain high levels of vitamins, minerals, proteins, fibres and health-promoting secondary metabolites with antioxidant, antibiotic, antidiabetic, anticancer, and other nutraceutical properties [2-6]. These could be valuable sources for alleviation of both malnutrition and non-communicable diseases in the general population.
In Cameroon, the commonly consumed AILVs include bitter leaf (Vernonia amygdalina), African vegetable nightshades (Solanum species), vegetable amaranths (Amaranthus species), fluted pumpkin (Cucurbita spp.), cowpea leaves (Vigna unguiculata), African eggplant (Solanum aethiopicum), moringa leaves (Moringa oleifera), sweet potato leaves (Ipomoea batatas) and cassava leaves (Manihot esculenta) [7]. Many of them are resilient, adaptive, and tolerate adverse climatic conditions more than exotic species such as cabbages, tomatoes and carrots. The availability of AILVs during the rainy season and their climate adaptability make them an attractive option for nutritional supplementation for those in need [6]. Therefore, they must be processed to maintain or improve their nutritional content, phytochemical content, organoleptic qualities, and long-term storage properties [2, 7]. One such processing technique is fermentation.
Fermented foods in Africa contain a broad range of plant-based products derived from maize, sorghum, millet, and cassava, among other sources [8]. Leafy vegetables are rarely fermented in Africa. Considering that the controlled fermentation of some AILVs with well-characterized LAB can improve bioactive metabolites, such as phenols and flavonoids, and enhance antioxidant activities [9-11], it is reasonable to assume that these types of vegetables are a good source of LAB growth. Some studies indicate that the most common LAB genera associated with fermented AILVs include Lactobacillus, Lactococcus, and Weissella, however, limited studies exist on the isolation and identification of indigenous LAB from AILVs. This study adds knowledge for these types of vegetables as a good source of LAB growth, increasing of their phytochemical content, and subsequently improving their antioxidant activities. It has been suggested that vegetables are a good source of LAB growth [12]. Generally, LAB are a group of Gram-positive, aerotolerant, acid-tolerant, non-sporulating rod or cocci organisms that play an important role in food fermentation by inhibiting pathogenic microorganisms [13]. Lactic acid bacteria use the Embden Meyer of Parnas (EMP) pathway to ferment carbohydrates and generate lactic acid as the final product [14]. However, limited information is available on the changes in phenolic content and antioxidant properties of AILVs fermented with different LAB strains isolated from the AILVs themselves. Moreover, the use of LAB (e.g., some L. plantarum strains) as starter cultures in vegetable fermentation increases the control of fermentation. Furthermore, the acidification of foods by organic acids produced by LAB strains and bacteriocins during fermentation can extend the shelf life and improve the safety of the products for consumption [9]. Therefore, the main objective of this study was to isolate, characterize, and identify LAB from fresh AILVs consumed in Cameroon and screen them for total phenolic content and antioxidant capacity upon fermentation.
2. Materials and methods
2.1. Sampling and preparation of materials
Four AILVs (AN, AL, FP and WL) were obtained from a vegetable farmer at Nkozoa (3.96667°N, 11.53333°W), a village in the Region of Yaounde, Cameroon. The plants were cultivated for 8-12 weeks. Hand-picking was used to harvest the vegetables, which were then shipped in sterile plastic sealed bags to the laboratory (Laboratory of Microbiology, LABO180), University of Yaounde 1, Cameroon for processing.
2.2. Isolation and identification of microorganisms
2.2.1. Isolation and purification of LAB from African indigenous leafy vegetables.
Prior to fermentation, LABs were isolated from the fresh leaves of AN, FP, AL and WL as the reported methods [15-17]. A mass of 10 g of each vegetable species was cut with sterilized scissors and immersed in a glass bottle containing 90 mL of physiological water. The leaves were macerated in physiological water for about 3 min until the color of the liquid changed. Furthermore, serial dilutions were made using sterilized physiological water before inoculation of the culture media. Then, a sequential decimal dilution of the homogenate was obtained. From the appropriate dilutions, 0.1 mL aliquots were spread plated on duplicate pre-dried surfaces of MRS (de Man, Rogosa, and Sharp) agar (TM MEDIA, Titan Biotech Ltd, India) plates. Inoculated plates were incubated under anaerobic condition at 37°C for 48 h. Subsequently, isolates that corresponded to lactic acid bacteria characteristics were purified by streaking method [18] on MRS medium.
2.3. Molecular identification of the LAB isolates
2.3.1. DNA extraction and purification
Genomic DNA was extracted from the isolates using the Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, Catalogue No. D6005), as described by Lane et al. [19]. The quality and quantity of the extracted DNA were subsequently measured using a Nanodrop (Thermo Scientific™ NanoDrop™ One Microvolume UV-Vis Spectrophotometer).
2.3.2. PCR amplification
The target region was amplified using OneTaq® Quick-Load® 2X Master Mix (NEB, Catalogue No.M0486). 27F-AGAGTTTGATCMTGGTCAG and 1492R- CGGTTACCTTGTTACGACT are the primers that were used for the reaction: The samples were then subjected to thermal cycling conditions (initial denaturation at 94℃ for 5 min, 3 series of denaturation, annealing and extension, final extension at 68℃ for 10minutes) using the Eppendorf Master cycler nexus gradient 230 PCR. Products were cleaned using an enzymatic method (ExoSAP).
2.3.3. DNA sequencing
The fragments were sequenced using the Nimagen, Brilliant Dye™ Terminator Cycle Sequencing Kit V3.1, and BRD3-100/1000 according to the manufacturer’s instructions. The labelled products were then cleaned using the ZR-96 DNA Sequencing Clean-up Kit (Catalogue No. D4053). The cleaned products were injected into the Applied Biosystems ABI 3500XL Genetic Analyser with a 50 cm array, using POP7 and sequence data were collected. BioEdit Sequence Alignment Editor (version 7.2.5) was used to analyse the files generated by the ABI 3500XL Genetic Analyzer and results were obtained by a BLAST search (NCBI), which were then submitted to the NCBI gene database.
2.4. Fermentation screening of African indigenous leafy vegetables
Fermentation screening was done by using the systematic replicate design [20] in other to select the best LAB strain that will enhance the total phenolic content and antioxidant capacities of the vegetables.
2.4.1. Preparation of leaves and fermentation bottles
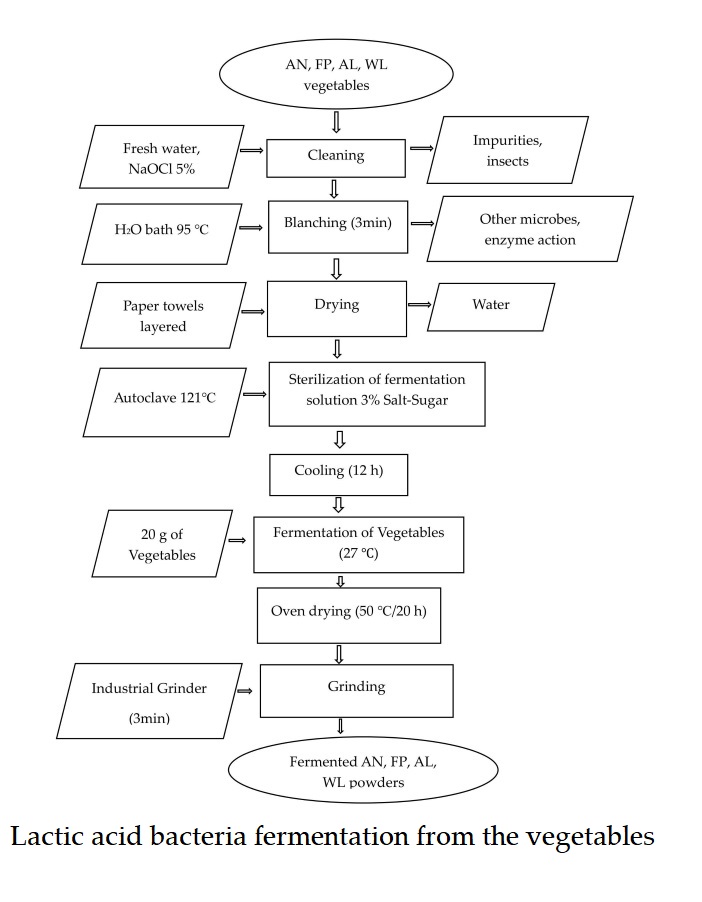
This was performed according to the method [9]. The AILVs (AN, FP, AL and WL) were procured from the same farmer at Nkozoa, Yaounde and transported in sterile sealed bags to the Microbiology Laboratory at the University of Yaounde 1. Freshly collected vegetables were detached from the stem and thoroughly washed under running tap water to remove impurities and insects (using hand gloves). The leaves were disinfected for 3 min with 5% sodium hypochlorite and rinsed with distilled water to remove excess chlorine from the leaves. The leaves were blanched in a water bath at 95 ℃ for 3 min, immersed in cool water, quickly transferred in separate colanders and allowed to dry (Fig. 1).
2.4.2. Starter cultures and fermentation of the African indigenous leafy vegetables
LAB were grown on MRS agar at 37 ℃ for 72 h. The strain reactivation was done by the following method [9]. Thereafter, 20 g of each vegetable leaves (AN, FP, AL and WL) was introduced in 60 mL of fermentation solution (3% Salt-Sugar) as described by [16]. The new media was inoculated with LAB isolates at a concentration of 106 CFU/mL and placed at 27 ℃ (Fig.1), for 0, 1, 2, 3 and 4 days. The control samples were not inoculated with LAB cultures. Fermentation was performed in duplicate for each LAB strain. Screening using the systematic replicate design [20], was used for the LAB fermentation process of the AILVs and shown in Table 1.
Table 1. Screening design for the fermentation of African indigenous leafy vegetables.
Coded LAB Isolate | Fermentation time/days | |||||
0 | 1 | 2 | 3 | 4 | ||
UnAN | 0 | F0AN | F1AN | F2AN | F3AN | F4AN |
UnAL | F0AL | F1AL | F2AL | F3AL | F4AL | |
UnFP | F0FP | F1FP | F2FP | F3FP | F4FP | |
UnWL | F0WL | F1WL | F2WL | F3WL | F4WL | |
AN | 1 | F0AN1 | F1AN1 | F2AN1 | F3AN1 | F4AN1 |
AL | F0AL1 | F1AL1 | F2AL1 | F3AL1 | F4AL1 | |
FP | F0FP1 | F1FP1 | F2FP1 | F3FP1 | F4FP1 | |
WL | F0WL1 | F1WL1 | F2WL1 | F3WL1 | F4WL1 | |
AN | 2 | F0AN2 | F1AN2 | F2AN2 | F3AN2 | F4AN2 |
AL | F0AL2 | F1AL2 | F2AL2 | F3AL2 | F4AL2 | |
FP | F0FP2 | F1FP2 | F2FP2 | F3FP2 | F4FP2 | |
WL | F0WL2 | F1WL2 | F2WL2 | F3WL2 | F4WL2 | |
AN | 3 | F0AN3 | F1AN3 | F2AN3 | F3AN3 | F4AN3 |
AL | F0AL3 | F1AL3 | F2AL3 | F3AL3 | F4AL3 | |
FP | F0FP3 | F1FP3 | F2FP3 | F3FP3 | F4FP3 | |
WL | F0WL3 | F1WL3 | F2WL3 | F3WL3 | F4WL3 | |
AN | 4 | F0AN4 | F1AN4 | F2AN4 | F3AN4 | F4AN4 |
AL | F0AL4 | F1AL4 | F2AL4 | F3AL4 | F4AL4 | |
FP | F0FP4 | F1FP4 | F2FP4 | F3FP4 | F4FP4 | |
WL | F0WL4 | F1WL4 | F2WL4 | F3WL4 | F4WL4 | |
AN | 5 | F0AN5 | F1AN5 | F2AN5 | F3AN5 | F4AN5 |
AL | F0AL5 | F1AL5 | F2AL5 | F3AL5 | F4AL5 | |
FP | F0FP5 | F1FP5 | F2FP5 | F3FP5 | F4FP5 | |
WL | F0WL5 | F1WL5 | F2WL5 | F3WL5 | F4WL5 | |
Un= control, AN: African nightshade, AL: amaranth leaf, FP: Fluted pumpkin, WL: water leaf, F0= zero-day fermentation, F1= 1day fermentation F2= 2days fermentation, F3= 3days fermentation, F4= 4 days fermentation.
2.5. Determination of pH
pH determination was done as per earlier methods [9, 21]. The pH of the brine, before and after mixing with leaves (10 mL), was sterilely taken from the bottles, and the pH values recorded using a digital pH meter (HANNA, instruments, HI12963) at 0, 1, 2, 3 and 4 days respectively.
2.6. Determination of phenolic compounds and antioxidant capacities of the fermented vegetables
After fermentation, the leaves were separated from the brine and oven-dried at 50 ℃ for 12 h, (Fig. 1) for the analyses of total phenolic content and antioxidant properties.
2.6.1. Extraction of free phenolic compounds
This was done following the method described by Dai et al. [22]. One gram of each fermented powder (AN, FP, AL and WL) was put to 30 mL of ethanol 70% (30:70 w/v). The mixture was agitated (IKA C-MAG HS 7 agitator) at 1500 rpm for 1h, then filtered using Whatman filter paper N°1 in the dark until the extraction solvent became clear. The residue was reserved for the extraction of bound phenolic compounds (BF). The extract (supernatant) was then collected and preserved at 4°C for subsequent use.
2.6.2. Extraction of bound phenolic compounds
The extraction of BF compounds was done by the following the method as Li et al. [23], using the residue obtained after the extraction of free phenolic compounds. A 40 mL of NaOH (4M) was added to the mixture and agitated for 4 h with the help of a magnetic agitator to hydrolyze the bound phenolic compounds. The pH was then adjusted to 2 with a pH meter using HCl 1M. Subsequently, 200 mL of ethanol 70% was added, the mixture was agitated for 24 h, and filtered using Whatman filter paper N° 1. The filtrate was evaporated using a rotary evaporator (BUCHI HB 140 water bath) at 40°C until complete evaporation of the extraction solvent. The content of the flask was collected in 40 mL of 70% ethanol and preserved at 4°C for subsequent use.
2.6.3. Determination of phenolic compounds
It was performed by the following method as described by Vinson et al. [24]. To 100µL of each extract, 2000 µL of distilled water and 200 µL of Folin-Ciocalteu reagent 2 N were added. After agitation and incubation for 5 min, 1000 µL of sodium carbonate solution of 10 % was added and stirred. The mixture was incubated at room temperature in the dark for 15 min, diluted to 1/10 and vortexed to obtain a homogeneous mixture. The absorbance was read at 700 nm against a blank tube containing the extraction solvent instead of the extract. The phenolic compound content of each sample was determined using a calibration curve with gallic acid as the standard. The experiments were done in triplicate and phenolic contents were calculated by using a calibration curve (OD= f (Cp)) with the following linear regression line equation:
Y= aX + b
Where,
(Y)= optical density (OD) of the solutions,
(X)= ponderal concentrations (Cp) of phenolic compounds,
(a)= slope of the curve,
(b)= intercept of the Y-axis.
2.6.4. Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity
The evolution of the antioxidant activity for the extracts (from LAB fermented AN, FP, AL and WL), was performed according to the protocol described by Lopez-Lutz et al. [25]. Into test tubes were introduced extract concentrations of 50 µL. A 3 mL of DPPH ethanolic solution 0.004 % (w/v) was added. After agitation, the tubes were kept in the dark at room temperature for 30 min and the absorbance of the reaction medium was read at 517 nm against a blank. DPPH in the absence of samples was used as a negative control, and hydroethanolic solvents (30/70) was as the blank. Gallic acid was used as the standard to compare the antiradical efficiency. The results were expressed as percentage as the inhibition of free radicals using the following formula:
2.6.5. Determination of ferric reducing antioxidant power assay (FRAP)
This was done following the method described by Benzie and Strain [26]. To 0.1 mL of the ethanolic extract (from LAB fermented AN, FP, AL and WL), 3 mL of freshly prepared FRAP reagent was added. After 5 min of incubation, the absorbance of the reaction medium was read at 593 nm against a blank. The FRAP activity was determined from the calibration curve using the regression equation of the plot. The ferric reducing power was expressed in mg of Fe2SO4/100g.
2.6.6. Determination of total antioxidant capacity (TAC)
The TAC content was determined as described by Prieto et al. [27]. A volume of 1 mL of the reagent solution (28 mM sodium phosphate, 0.6 M sulfuric acid and 4 mM ammonium molybdate) was mixed with 0.1 mL of the vegetable extracts (from LAB fermented AN, FP, AL and WL) in Eppendorf tubes. All samples were incubated in a water bath at 95°C for 90 min and cooled to 25°C. The absorbance of the green phosphomolybdenum complex was recorded at 695 nm against a blank. The higher the absorbance of the extract the more effective the antioxidant compound. Results were expressed as gallic acid equivalents.
2.7. Data analysis
Data entry management and preliminary summaries were performed using Microsoft Excel spreadsheet, and SPSS (version 20). Data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s HSD test at 95% confidence interval (p ≤ 0.05). Percentage (%) variation was used to screen for the effects of LAB fermentation of the vegetables for pH, total polyphenols and antioxidant properties. BioEdit Sequence Alignment Editor (version 7.2.5) was used to analyze the files generated by the ABI 3500XL Genetic Analyzer and results were obtained by a BLAST search (NCBI) which were then submitted to the NCBI Gene bank database.
3. Results and discussion
3.1. Phenotypic and molecular identification of lactic acid bacteria from African indigenous leafy vegetables
All strains isolated from AN, WL, AL and FP were classified as Gram-positive and catalase-negative (Table 2), with three rod shaped and two cocci shaped. The sequence length for all strains ranged from 1001 to 1531 base pairs (Fig. 2, Table 2).
Figure 2. A photographic image of an agarose gel indicating the amplification of the 16S target region.
Table 2. Phenotypic and molecular identification of LAB from AILVs.
Isolates | Origin | Phenotypic characterization | Molecular characterization | ||||
Cell Shape | Gram Status | Catalase | Closest Relatives | S.L /(bp) | Accession No. | ||
AN | Rods | + | - | 1531 | PV666101.1 | ||
WL | Rods | + | - | Lactiplantibacillus pentosus | 1519 | PV666098.1 | |
L2 | AL | Cocci | + | - | Leuconostoc mesenteroides | 1522 | PV666097.1 |
L31 | AN | Rods | + | - | Lactiplantibacillus plantarum | 1001 | PV666100.1 |
L33 | FP | Cocci | + | - | Leuconostoc mesenteroides | 1512 | PV666099.1 |
S/L=Sequence length | |||||||
These were submitted to the NCBI Gene Bank database for accession. Lactiplantibacillus plantarumL52 and Lactiplantibacillus plantarumL31 were identified in fresh AN, Lactiplantibacillus pentosusL7 from WL, Leuconostoc mesenteroidesL2 from AL leaves and Leuconostoc mesenteroidesL33 from FP. Similar strains of LAB have been identified by [15, 16] from the fermented broth of African nightshade, vegetable amaranth and cowpea, where Lactobacillus plantarum dominated vegetable amaranth and nightshade fermentation.
3.2. Fermentation screening for phenolics and antioxidant capacities in the four vegetables
The bound and free phenolics ranged from (247.51 ± 12.10 to 343.65 ± 13.69) mgEAG/100g in WL and AN, respectively and from (787.22 ± 22.69 to 932.27 ± 13.24) mgEAG/100g in the raw WL and AN, respectively (Table 3). There were no significant differences (p ≤ 0.05) in the DPPH radical scavenging activity of all the vegetables. There were significant differences (p ≤ 0.05) in the TAC and FRAP activity of the raw vegetables. The highest TAC (1755.35 ± 27.98 mgEAG/100g) and FRAP (3085.56 ± 39.47 mgFeSO4/100g) activities were recorded in AN when compared to the other vegetables FP, WL and AL. This makes AN, the best vegetable because of its improved total phenolic content and antioxidant activity. The mean values of phenolic content and antioxidant activities obtained in this study are twice higher than those obtained by reported data [28] from raw and cooked AL and AN.
Table 3. Phenolic content and antioxidant capacities of the raw vegetables.
|
Veg |
BP/mgEAG/100g |
FrP/mgEAG/100g |
TAC/mgEAG/100g |
DPPH/% |
FRAP/mgFeSO4/100g |
|
FP |
320.72±21.03a |
834.55±23.62a |
1738.89±19.94a |
89.32±3.44a |
1406.45±29.25bc |
|
AN |
343.65±13.69a |
932.27±13.24a |
1755.35±27.98a |
87.90±3.74a |
3085.56±39.47a |
|
AL |
290.33±10.98a |
876.82±17.36a |
1256.72±35.18b |
86.79±4.13a |
1574.44±31.71b |
|
WL |
247.51±12.10a |
787.27±22.69a |
910.59±34.40b |
87.52±4.10a |
1271.11±33.02c |
|
Mean ± SD (n=3), values
within the same column followed by the same superscripts are not
significantly different (P≤0.05). |
|||||
3.3. Effect of lactic acid bacteria strains on pH
The changes in pH during LAB fermentation from day 0 to day 4 in all the four vegetables (AN, FP, AL and WL) are shown in Table 4. The pH value dropped from 6.39 ± 0.006 to 3.82 ± 0.006 at the end of the fermentation period in the starter-culture-inoculated L. plantarumL52, while the pH values in the control fermentation dropped from 6.49 ± 0.012 to 4.20 ± 0.000 in the vegetables. In addition, compared to the un-inoculated vegetables, the vegetables inoculated with L. plantarumL52 (Fig.3) showed the most significant (P ≤ 0.05) pH lowering ability. The ability of bacteria to acidify during fermentation is reflected in the pH.
Table 4. Changes in pH during 0 – 4 days lactic acid bacteria fermentation of the vegetables.
Blocks | Sample | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |
Un | BLOCK 1 | FP | 6.39±0.006n | 4.76±0.006i | 4.38±0.006c | 4.20±0.000a | 4.20±0.000a |
AN | 6.41±0.006o | 5.01±0.000m | 4.66±0.006g | 4.73±0.006h | 4.86±0.000k | ||
AL | 6.49±0.012p | 4.96±0.000l | 4.62±0.000f | 4.54±0.017e | 4.53±0.001e | ||
WL | 6.49±0.006p | 4.78±0.000j | 4.41±0.000d | 4.33±0.006b | 4.38±0.000c | ||
L2 | BLOCK 2 | FP | 6.23±0.006p | 4.53±0.006i | 4.30±0.000g | 4.16±0.006c | 4.14±0.006b |
AN | 6.33±0.006r | 4.93±0.006n | 4.60±0.000j | 4.43±0.006h | 4.63±0.006k | ||
AL | 6.31±0.000q | 4.91±0.006m | 4.15±0.000bc | 4.12±0.000a | 4.28±0.006f | ||
WL | 6.06±0.006o | 4.76±0.006l | 4.26±0.006e | 4.24±0.006d | 4.30±0.006g | ||
L31 | BLOCK 3 | FP | 6.25±0.006m | 4.56±0.006g | 4.33±0.006e | 4.10±0.000a | 4.11±0.000a |
AN | 6.40±0.000o | 4.78±0.006k | 4.62±0.006h | 4.61±0.006h | 4.62±0.000h | ||
AL | 6.37±0.006n | 4.75±0.000j | 4.30±0.006d | 4.17±0.006b | 4.20±0.000c | ||
WL | 5.90±0.000l | 4.73±0.000i | 4.37±0.006f | 4.34±0.006e | 4.36±0.006f | ||
L33 | BLOCK 4 | FP | 6.24±0.000m | 4.55±0.000i | 4.35±0.006e | 4.19±0.006b | 4.05±0.006a |
AN | 6.40±0.000n | 4.85±0.006k | 4.50±0.000h | 4.49±0.012h | 4.49±0006h | ||
AL | 6.41±0.006n | 4.71±0.000j | 4.23±0.006c | 4.46±0.012g | 4.40±0.000f | ||
WL | 5.92±0.006l | 4.71±0.000j | 4.33±0.012e | 4.03±0.000a | 4.25±0.000d | ||
L52 | BLOCK 5 | FP | 6.24±0.000p | 4.51±0.000k | 4.19±0.000j | 4.00±0.000e | 3.90±0.006b |
AN | 6.36±0.006q | 4.95±0.006n | 4.05±0.006g | 3.92±0.012d | 4.05±0.000g | ||
AL | 5.91±0.000o | 4.93±0.006m | 4.16±0.000i | 4.03±0.000f | 4.00±0.000e | ||
WL | 6.36±0.000r | 4.68±0.006l | 4.12±0.000h | 3.82±0.006a | 3.92±0.006c | ||
L7 | BLOCK 6 | FP | 6.19±.006k | 4.65±0.006gh | 4.37±0.006ef | 4.18±0.006bcd | 4.14±0.006bc |
AN | 6.31±0.006k | 4.92±0.000i | 4.57±0.006g | 4.52±0.000fg | 4.70±0.266gh | ||
AL | 6.20±0.006k | 4.93±0.006i | 4.28±0.006cde | 4.07±0.000b | 4.27±0.006cde | ||
WL | 5.65±0.006j | 4.78±0.006hi | 4.34±0.012def | 3.87±0.006a | 4.37±0.006ef | ||
Mean±SD (n=3) values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7 | |||||||
Figure 3. pH changes in African nightshade during a 0-4 days fermentation.
This makes L. plantarumL52 the most functionally effective strain of L. plantariumL31, L. mesenteroidesL33, L. mesenteroidesL2 and L. pentosusL7 in terms of pH reduction. Within one day of fermentation, the pH was lower (4.51± 0.000 to 4.95±0.006) when compared to the pH of 5.20 in the starter-culture-inoculated African black nightshade and African spider plant observed by [9] at three days and [16] at one day. Vegetables inoculated with L. plantarumL52 had significantly lower the pH values (3.82 ± 0.006, 3.92 ± 0.012 and 3.90 ± 0.006) after three and four days of fermentation for WL AN and FP, respectively. The inoculum contributed to the growth of lactic acid bacteria in starter culture-inoculated AILVs thus producing various organic acids, including lactic acid which contributed to the lower pH observed in these vegetables [14] production. This study also concurs with the reported findings [16], as Lactiplantibacillus starter culture was the predominant LAB that led to a significant reduction in pH in African black nightshade and African Spider plant.
3.4. Effect of lactic acid bacteria strains on bound phenolic (BF) content
There were significant (p ≤ 0.05) changes in the bound phenolic content in all the four AILVs fermented by LAB strains during the 0-4day period (Table 5). There was a general decrease in the BF content in all the starter culture fermented vegetables from day 1 to day 4, with the greatest impact observed from day 2 to day 3 (268.23 ± 0.48 to174.03 ± 0.14) mgEAG/100g; (251.38 ± 0.48 to 88.67 ± 0.14) mgEAG/100g in AN, AL and WL fermented by L. plantarumL31 and L. mesenteroidesL33, respectively.
Table 5. Influence of fermentation by different lactic acid bacteria strains on bound phenolic content of the vegetables.
LAB strains | Blocks | Sample | Fermentation time/days | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |||
Un | BLOCK 1 | FP | 423.48±5.32r | 313.26±5.67l | 240.06±6.27f | 259.67±5.48g | 216.30±5.01d |
AN | 405.25±5.14q | 388.67±5.72p | 311.33±5.48k | 331.49±5.66m | 374.59±5.26o | ||
AL | 367.13±3.87n | 301.66±5.78j | 240.33±4.96f | 206.35±3.46c | 279.28±3.56i | ||
WL | 277.35±6.48h | 216.57±6.48d | 151.66±5.16b | 225.41±6.83e | 143.09±5.48a | ||
L2 | BLOCK 2 | FP | 266.30±0.48k | 300.83±0.03m | 174.86±0.01e | 168.23±0.12c | 254.70±0.48j |
AN | 248.34±0.48i | 404.42±0.83q | 248.90±0.48i | 265.75±0.48k | 316.30±0.48o | ||
AL | 293.09±0.48l | 345.86±0.48p | 174.03±0.14e | 216.30±0.02g | 309.94±0.83n | ||
WL | 171.55±0.12d | 186.46±0.83f | 148.34±0.14a | 162.71±0.48b | 239.23±0.48h | ||
L31 | BLOCK 3 | FP | 311.05±2.53m | 267.13±0.96j | 173.20±0.12c | 223.76±0.12e | 275.97±0.14k |
AN | 377.90±014q | 284.53±0.48l | 268.23±0.48j | 256.63±0.48i | 367.40±0.48p | ||
AL | 322.10±1.27n | 274.31±0.14k | 213.81±0.12d | 174.03±0.14c | 342.82±0.48o | ||
WL | 242.27±0.48g | 249.45±0.16h | 127.62±0.12b | 88.67±0.14a | 232.60±0.48f | ||
BLOCK 4 | FP | 269.34±0.83k | 267.40±0.48jk | 214.64±0.12e | 155.80±0.12b | 238.67±0.16f | |
AN | 363.54±2.01p | 308.29±0.16m | 251.38±0.48h | 246.69±0.48g | 326.52±0.16n | ||
AL | 304.42±2.53l | 265.19±0.14j | 216.30±0.14e | 172.65±0.48c | 338.40±0.48o | ||
WL | 171.27±0.48c | 206.35±0.14d | 239.50±0.14f | 144.20±0.18a | 256.08±0.14i | ||
L52 | BLOCK 5 | FP | 225.97±0.48g | 275.14±0.12k | 174.03±0.12b | 188.12±0.12d | 274.31±0.12k |
AN | 374.59±0.14q | 334.53±0.48p | 241.16±0.14i | 308.01±1.26n | 271.27±0.48j | ||
AL | 210.50±0.14f | 323.76±0.48o | 174.59±0.48b | 174.86±0.14b | 297.51±0.14m | ||
WL | 291.99±1.27l | 228.45±0.48h | 183.98±0.14c | 164.92±0.14a | 198.07±0.14e | ||
L7 | BLOCK 6 | FP | 291.99±0.48l | 293.37±0.12l | 218.78±0.12h | 188.67±1.00e | 214.92±0.48g |
AN | 324.86±0.14o | 372.10±0.14q | 270.44±0.48j | 277.35±0.48k | 330.39±0.48p | ||
AL | 162.98±0.48c | 319.06±0.16n | 187.29±0.16e | 147.79±0.48b | 310.77±0.83m | ||
WL | 263.81±1.00i | 217.68±0.48h | 203.31±0.46f | 139.50±0.48a | 170.17±0.48d | ||
Mean±SD(n=3) values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7 | |||||||
The percentage variation in BF content during 0–4-day fermentation by the LAB strains in all four AILVs is shown in Table 6. When compared to control vegetables, vegetables inoculated with L. plantarumL31 showed the highest decrease in BF, -44.3% for FP, -32.1% for AN, -33.6% for AL and -63.4% for WL. This makes L. plantarumL31 the best LAB among strains L. plantarumL52, L. mesenteroidesL33, L. mesenteroidesL2 and L. pentosusL7 in terms of bound phenols reduction during fermentation. The reduction in bound phenols during fermentation suggests that they are broken down into their free forms [29]. Through the metabolic activities of LAB, fermentation also induces structural breakdown of the cell wall, which leads to the synthesis of various bioactive compounds [30], enhanced bioavailability, and the release of bound phenolics to free phenolics [29].
Table 6. Percentage variation in bound phenol content during fermentation.
Vegetable | LAB strains | BP Variation During Fermentation/ (%) | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | ||
FP | Un | 0.0 | -26.0 | -43.3 | -38.7 | -48.9 |
L2 | 0.0 | 13.0 | -34.3 | -36.8 | -4.4 | |
L31 | 0.0 | -14.1 | -44.3 | -28.1 | -11.3 | |
L33 | 0.0 | -0.7 | -20.3 | -42.2 | -11.4 | |
L52 | 0.0 | 21.8 | -23.0 | -16.7 | 21.4 | |
L7 | 0.0 | 0.5 | -25.1 | -35.4 | -26.4 | |
AN | Un | 0.0 | -4.1 | -23.2 | -18.2 | -7.6 |
L2 | 0.0 | 62.8 | 0.2 | 7.0 | 27.4 | |
L31 | 0.0 | -24.7 | -32.1 | -29.0 | -2.8 | |
L33 | 0.0 | -15.2 | -30.9 | -32.1 | -10.2 | |
L52 | 0.0 | -10.7 | -35.6 | -17.8 | -27.6 | |
L7 | 0.0 | 14.5 | -16.8 | -14.6 | 1.7 | |
AL | Un | 0.0 | -17.8 | -34.5 | -43.8 | -23.9 |
L2 | 0.0 | 18.0 | -40.6 | -26.2 | 5.7 | |
L31 | 0.0 | -14.8 | -33.6 | -46.0 | 6.4 | |
L33 | 0.0 | -12.9 | -28.9 | -43.3 | 11.2 | |
L52 | 0.0 | 53.8 | -17.1 | -16.9 | 41.3 | |
L7 | 0.0 | 95.8 | 14.9 | -9.3 | 90.7 | |
WL | Un | 0.0 | -21.9 | -45.3 | -18.7 | -48.4 |
L2 | 0.0 | 8.7 | -13.5 | -5.2 | 39.5 | |
L31 | 0.0 | 3.0 | -63.4 | -47.3 | -4.0 | |
L33 | 0.0 | 20.5 | 39.8 | -15.8 | 49.5 | |
L52 | 0.0 | -21.8 | -37.0 | -43.5 | -32.2 | |
L7 | 0.0 | -17.5 | -22.9 | -47.1 | -35.5 | |
Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strain L52=L. plantarum L52, Strain L7=L. pentosusL7 | ||||||
3.5. Effect of lactic acid bacteria strains on free phenolic content of the vegetables
There were significant differences (p≤0.05) in the free phenolic (FrP) content of all the vegetables (AN, FP, AL, WL) fermented with the LAB strains under study, (Table 7). This was particularly observed in FP and AN fermented with LAB strain L. mesenteroidesL2 (781.82 ± 0.79 to 1228.18 ± 0.79, 858.64 ± 0.79 to 986.82 ± 0.79) mgEAG/100g, L. plantarumL31(737.27 ± 0.79 to 1152.27 ± 0.00, 922.72 ± 0.79 to 1021.36 ± 0.00) mgEAG/100g and L. mesenteroidesL33 (856.82 ± 2.84 to 1253.64 ± 0.79, 964.55 ± 2.03 to1070.0 ± 0.79) mgEAG/100g for four days and three days respectively.
LAB strains | Blocks | Sample | Fermentation time/days | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |||
Un | BLOCK 1 | FP | 913.64±0.00p | 918.64±0.79q | 905.91±0.79o | 926.36±0.79r | 882.27±0.00m |
AN | 894.55±0.00n | 843.18±2.03k | 860.46±0.00l | 829.09±0.00j | 810.0±1.36h | ||
AL | 810.46±0.79h | 590.46±0.00f | 731.82±0.79g | 501.82±0.00b | 580.0±4.38e | ||
WL | 891.82±1.36n | 466.36±0.00a | 551.36±0.79d | 816.82±0.00i | 527.27±2.08c | ||
BLOCK 2 | FP | 781.82±.79h | 864.55±4.92j | 1131.36±0.79q | 1117.73±2.84p | 1228.18±0.79r | |
AN | 858.64±.79i | 945.91±0.79n | 945.0±0.00n | 986.82±0.79o | 932.27±2.08m | ||
AL | 923.64±3.15l | 668.64±0.79g | 510.91±0.79b | 502.27±1.57a | 536.36±0.79d | ||
WL | 913.64±1.36k | 545.46±0.00e | 549.55±0.00e | 600.45±0.79f | 522.73±0.79c | ||
L31 | BLOCK 3 | FP | 737.27±0.79f | 915.91±0.79i | 1133.64±0.79p | 1117.27±0.79o | 1152.27±0.00q |
AN | 922.72±0.79jk | 925.00±2.08k | 965.46±1.36m | 1021.36±0.00n | 936.36±4.38l | ||
AL | 910.46±0.79h | 919.09±0.00ij | 569.55±0.79b | 642.27±0.00e | 638.64±2.08e | ||
WL | 871.82±0.79g | 611.82±1.58d | 569.55±0.79c | 520.91±0.79a | 568.64±0.79b | ||
L33 | BLOCK 4 | FP | 856.82±2.84i | 983.18±1.36l | 1074.55±2.36p | 1253.64±0.79r | 1095.45±0.79q |
AN | 1020±1.36n | 964.55±2.03k | 1070.0±0.79p | 1032.27±0.00o | 1002.27±3.61m | ||
AL | 905.91±0.79j | 818.64±.0.79h | 537.27±1.36c | 738.18±0.79g | 643.18±1.57e | ||
WL | 691.36±0.00f | 538.18±0.79c | 500.0±0.79a | 525.45±0.79b | 579.54±1.36d | ||
L52 | BLOCK 5 | FP | 882.73±0.79j | 939.55±0.00m | 1129.55±0.79p | 1190.91±0.79s | 1164.09±0.79r |
AN | 1135.46±0.79q | 922.73±1.58k | 990.46±2.08o | 937.27±0.79m | 976.36±0.00n | ||
AL | 926.82±0.79l | 706.82±0.79g | 519.55±1.36c | 545.45±0.00d | 850.91±1.36i | ||
WL | 777.73±0.79h | 595.00±2.03e | 511.36±0.00b | 500.45±0.00a | 700.91±1.36f | ||
L7 | BLOCK 6 | FP | 991.36±0.00o | 1047.73±0.79p | 1142.73±1.36r | 1060.0±0.79q | 1317.27±0.00s |
AN | 881.36±0.79i | 942.27±0.00l | 1015.0±2.84n | 933.18±2.08k | 975.0±1.36m | ||
AL | 897.27±1.36j | 511.82±0.79d | 460.46±0.79b | 500.91±3.43c | 810.45±0.79g | ||
WL | 823.18±0.79h | 591.82±0.00f | 380.91±0.79a | 497.73±0.00c | 563.18±0.00e | ||
Mean±SD(n=3) values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7
Table 8 illustrates the percentage variation in free phenolic (FrP) content during the fermentation of the four AILVs from day 0 to day 4. There was a steady percentage increase in the FrP content of FP (43% and 51.5%) and AN (14.9% and 10.7%) from day 0 to day 3 fermented with LAB strains L. mesenteroidesL2 and L. plantarumL31, respectively. Compared to the control vegetables, vegetables inoculated with L. plantarum L31 showed the highest release of FrP. This was observed in the FP (56.3% increase from day 0 to day 4) and AN (10.7% increase from day 0 to day 3) groups. The increase in FrP content during the fermentation of AN and FP from day 1 to day 3 suggests that the metabolism by L. plantarumL31 let to the breakdown of the plant matrix, releasing phenolic compounds, [29]. Through metabolic activities of microbes, fermentation also induces structural breakdown of the cell wall, which leads to the synthesis of various bioactive compounds [30], enhanced bioavailability and the release of bound phenolics to free phenolics [29]. The trend in this study corroborates with the findings of Irakoze et al. [10] who showed that both LAB starter culture-inoculated African black nightshade and African spider plant showed significantly higher free phenolic compounds (10.96 mg/g QE and 6.67 mg/g QE, respectively) than uninoculated vegetables. Furthermore, Degrain et al. [9] showed that the concentration of free phenols in fermented nightshade product increased from 6007.8 mg/kg (raw leaves) to 8638.0, 8246.5, 8016.8, 5681.5 and 3822.5 mg/kg after fermenting with L. plantarum (17a), W. cibaria (21), L. pseudomesenteroides (56), W. cibaria (64) and L. plantarum (75). Similarly, fermentation with LAB strains increased the total phenolic compounds in kiwi fruit [31]. It has been shown that LAB strains including L. plantarum possess β-glucosidase enzymes that can hydrolyze the flavonoid conjugates during fermentation and influence the bioavailability of polyphenols [31].
Table 8. Percentage variation in free phenolic content during fermentation.
LAB Strains | FrP variation during fermentation / (%) | |||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | ||
FP | Un | 0.0 | 0.5 | -0.8 | 1.4 | -3.4 |
L2 | 0.0 | 10.6 | 44.7 | 43.0 | 57.1 | |
L31 | 0.0 | 24.2 | 53.8 | 51.5 | 56.3 | |
L33 | 0.0 | 14.7 | 25.4 | 46.3 | 27.9 | |
L52 | 0.0 | 6.4 | 28.0 | 34.9 | 31.9 | |
L7 | 0.0 | 5.7 | 15.3 | 6.9 | 32.9 | |
AN | Un | 0.0 | -5.7 | -3.8 | -7.3 | -9.5 |
L2 | 0.0 | 10.2 | 10.1 | 14.9 | 8.6 | |
L31 | 0.0 | 0.2 | 4.6 | 10.7 | 1.5 | |
L33 | 0.0 | -5.4 | 4.9 | 1.2 | -1.7 | |
L52 | 0.0 | -18.7 | -12.8 | -17.5 | -14.0 | |
L7 | 0.0 | 6.9 | 15.2 | 5.9 | 10.6 | |
AL | Un | 0.0 | -27.1 | -9.7 | -38.1 | -28.4 |
L2 | 0.0 | -27.6 | -44.7 | -45.6 | -41.9 | |
L31 | 0.0 | 0.9 | -37.4 | -29.5 | -29.9 | |
L33 | 0.0 | -9.6 | -40.7 | -18.5 | -29.0 | |
L52 | 0.0 | -23.7 | -43.9 | -41.1 | -8.2 | |
L7 | 0.0 | -43.0 | -48.7 | -44.2 | -9.7 | |
WL | Un | 0.0 | -47.7 | -38.2 | -8.4 | -40.9 |
L2 | 0.0 | -40.3 | -39.9 | -34.3 | -42.8 | |
L31 | 0.0 | -29.8 | -33.1 | -40.3 | -34.8 | |
L33 | 0.0 | -22.2 | -27.7 | -24.0 | -16.2 | |
L52 | 0.0 | -23.5 | -34.2 | -35.7 | -9.9 | |
L7 | 0.0 | -28.1 | -53.7 | -39.5 | -31.6 | |
Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strain L52=L. plantarum L52, Strain L7=L. pentosusL7 | ||||||
3.6. Effect of lactic acid bacteria strains on the total antioxidant capacity
Significant (p ≤ 0.05) changes in TAC were observed in all the four vegetables fermented with LAB strains L. mesenteroidesL2, L. plantarumL31, L. mesenteroidesL33, L. plantarumL52 and L. pentosusL7 (Table 9). The highest TAC activity was recorded during a three-day fermentation in all the vegetables (2619.31 ± 2.51 mgEAG/100g for FP, 2544.16 ± 1.65 mgEAG/100g for AN and 2176.08 ± 0.95 mgEAG/100g for WL) fermented with L. plantarumL31 except for AL. This observation differs from that of Zhao et al. [32] who recorded the highest increase in TAC in jujube-wolfberry LAB fermented composite juice after 2 days. The increase in TAC during the first three days of fermentation may be associated with the release of phenolic compounds during fermentation [9].
The percentage variation in TAC activity during fermentation from day 0 to day 4 in all the four AILVs (AN, FP, AL and WL) is illustrated in Table 10. Compared to the control vegetables, vegetables inoculated with L. plantarumL31 showed a higher percentage increase in TAC activities in AN (24.9%), WL (140%) and FP (47.5%) from day 0 to day 3 and AL (45.5%) from day 0 to day 2. This observation makes L. plantarumL31 the best LAB amongst strains L. plantarumL52, L. mesenteroidesL33, L. mesenteroidesL2 and L. pentosusL7 in terms of TAC activity during fermentation. The increased TAC activity observed in L. plantarumL31 ferments can be attributed to the intracellular antioxidant enzyme system. Additionally, Abduxukur et al. [33], found a similar trend in the increase of TAC in LAB starter fermented dairy products. However, Kaprasob et al., [34] showed a decrease in TAC activity in cashew apple juice and explained that this could be due to the oxidation of phenolic compounds.
Table 9. Influence of fermentation by lactic bacteria strain on total antioxidant capacity of the vegetables.
Blocks | Sample | Fermentation Time/Days | |||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |||
Un | BLOCK 1 | FP | 1624.25±1.65l | 1590.78±0.95i | 1490.40±0.95h | 1673.07±0.95n | 1601.21±1.65j |
AN | 1692.27±0.9o | 1452.00±0.95f | 1650.03±2.51m | 1612.73±1.65k | 1624.25±1.65l | ||
AL | 1467.91±0.00g | 843.66±0.95a | 1425.67±2.51e | 989.03±8.55c | 1428.41±1.65e | ||
WL | 894.68±0.95b | 1260.01±0.95d | 1466.26±1.65g | 1739.44±1.65p | 1486.56±2.51h | ||
L2 | BLOCK 2 | FP | 1896.32±2.51q | 1526.60±2.51i | 1907.30±1.65r | 1907.84±0.95r | 1671.97±4.3m |
AN | 1642.90±0.95l | 1540.32±1.65j | 1360.94±1.65g | 1737.79±1.65n | 1788.26±0.95o | ||
AL | 1018.10±0.95b | 709.27±1.65a | 1442.13±2.51h | 1818.43±1.65p | 1328.03±2.85f | ||
WL | 1094.35±0.00c | 1133.30±0.95d | 1306.64±3.29e | 2416.90±3.43s | 1624.25±3.29k | ||
BLOCK 3 | FP | 1776.19±3.43j | 1775.64±1.65j | 1563.91±0.95g | 2619.31±2.51s | 2387.82±1.65q | |
AN | 2036.20±2.51m | 1647.83±0.95h | 2282.50±1.65p | 2544.16±1.65r | 1889.19±1.65l | ||
AL | 1285.24±1.65c | 1007.68±.95b | 1869.99±2.51k | 1418.54±1.65e | 1552.93±2.51f | ||
WL | 906.75±2.85a | 1390.57±1.65d | 2104.774±4.35n | 1724.63±1.65i | |||
L33 | BLOCK 4 | FP | 1861.77±0.95l | 1196.38±1.65g | 2356.56±1.65r | 2272.63±3.29q | 1799.78±2.51j |
AN | 2105.87±0.95o | 1610.53±2.51i | 1295.67±0.95h | 2036.75±0.9m | 2075.15±1.65n | ||
AL | 1136.04±0.95d | 986.83±0.95b | 1295.67±2.51h | 1835.98±0.95k | 1864.51±1.65l | ||
WL | 693.36±0.95a | 1188.15±1.65f | 1084.48±1.65c | 2198.57±1.65p | 1154.14±0.95e | ||
L52 | BLOCK 5 | FP | 1628.09±0.95k | 1758.09±3.43n | 2370.27±1.90t | 2170.60±1.65r | 2110.26±2.5q |
AN | 1658.26±0.95m | 2044.98±0.95p | 1295.12±1.65g | 1643.99±1.65l | 1185.96±2.51e | ||
AL | 1172.24±0.95d | 900.16±1.65a | 1304.44±0.95h | 1589.69±1.65j | 1246.85±2.51f | ||
WL | 990.67±1.66b | 1017.55±0.95c | 2230.39±0.95s | 1918.27±1.90o | 1558.42±1.65i | ||
L7 | BLOCK 6 | FP | 2209.54±3.43p | 1419.09±3.43f | 1827.21±0.95n | 1642.35±1.65i | 2618.21±1.65q |
AN | 1863.41±0.95o | 1610.53±0.95h | 1806.36±0.95l | 1653.87±1.65j | 1824.47±3.43n | ||
AL | 1419.09±0.95f | 786.07±0.95a | 1303.35±0.00d | 1315.41±2.51e | 1734.50±1.65k | ||
WL | 1142.07±1.65b | 1517.28±0.00g | 1224.90±0.95c | 1731.21±1.65k | 1816.24±5.2m | ||
Mean ± SD (n=3) values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7
Table 10. Percentage variation in total antioxidant capacity during fermentation.
Vegetable | LAB strains | TAC variation during fermentation /(%) | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | ||
FP | Un | 0.0 | -2.1 | -8.2 | 3.0 | -1.4 |
L2 | 0.0 | -19.5 | 0.6 | 0.6 | -11.8 | |
L31 | 0.0 | 0.0 | -12.0 | 47.5 | 34.4 | |
L33 | 0.0 | -35.7 | 26.6 | 22.1 | -3.3 | |
L52 | 0.0 | 8.0 | 45.6 | 33.3 | 29.6 | |
L7 | 0.0 | -35.8 | -17.3 | -25.7 | 18.5 | |
AN | Un | 0.0 | -14.2 | -2.5 | -4.7 | -3.9 |
L2 | 0.0 | -6.2 | -17.2 | 5.8 | 8.9 | |
L31 | 0.0 | -19.1 | 12.1 | 24.9 | -7.2 | |
L33 | 0.0 | -23.5 | -38.5 | -3.3 | -1.5 | |
L52 | 0.0 | 23.3 | -21.9 | -0.9 | -28.3 | |
L7 | 0.0 | -13.6 | -3.1 | -11.2 | -1.9 | |
AL | Un | 0.0 | -42.5 | -2.9 | -32.6 | -2.7 |
L2 | 0.0 | -30.3 | 41.6 | 78.6 | 30.4 | |
L31 | 0.0 | -21.6 | 45.5 | 10.4 | 20.8 | |
L33 | 0.0 | -13.1 | 14.1 | 61.6 | 64.1 | |
L52 | 0.0 | -23.2 | 11.3 | 35.6 | 6.4 | |
L7 | 0.0 | -44.6 | -8.2 | -7.3 | 22.2 | |
WL | Un | 0.0 | 40.8 | 63.9 | 94.4 | 66.2 |
L2 | 0.0 | 3.6 | 19.4 | 120.9 | 48.4 | |
L31 | 0.0 | 53.4 | 132.1 | 140.0 | 90.2 | |
L33 | 0.0 | 71.4 | 56.4 | 217.1 | 66.5 | |
L52 | 0.0 | 2.7 | 125.1 | 93.6 | 57.3 | |
L7 | 0.0 | 32.9 | 7.3 | 51.6 | 59.0 | |
Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strain L52=L. plantarum L52, Strain L7=L. pentosusL7 | ||||||
3.7. Effect of lactic acid bacteria strains on 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
The radical scavenging activity (RSA) of the LAB fermented vegetables was significantly (p ≤ 0.05) different throughout the fermentation period with values decreasing as the fermentation time increased (Table 11). However, significant increases (from 85.03 ± 1.07% to 90.65 ± 1.07% and from 85.71 ± 1.45% to 92.28 ± 1.13%) in RSA were observed in AN fermented with L. plantarumL31 and L. mesenteroideL33 from day 0 to day 3, respectively. The study by Irakoze et al. [10] contrasts with the present study as they recorded a lower RSA of between 60% to 80% in LAB starter cultured African nightshade and spider plant. Table 12 illustrates the percentage variation in RSA during fermentation from day 0 to day 4 in all the four AILVs. When compared to the control vegetables, those inoculated with L. mesenteroidesL33 showed the most improved RSA. This was particularly observed in AN (from 0% to 7.7%) within 3 days fermentation. The higher percentage increase of RSA observed in L. mesenteroidesL33 starter cultured AN from day 0 to day 3 matches the high antioxidant activity noted in apple juice fermented with L. plantarum [35]. The increase in phytochemical content during fermentation has been attributed to the increase in antioxidant capacity of fermented foods [36, 37]. Overall, fermentation plays a significant role in improving and maintaining the antioxidant capacity of food through the production of important phenolic compounds such as quercetin [36].
LAB strains | Blocks | Sample | Fermentation Time/Days | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |||
Un | BLOCK 1 | FP | 90.22±1.13o | 68.85±1.00h | 76.88±1.07i | 76.96±1.26i | 83.66±1.13k |
AN | 93.18±1.00q | 90.18±1.07o | 84.47±1.07l | 78.46±1.07j | 85.29±1.20m | ||
AL | 91.93±1.20p | 49.03±1.13f | 86.87±1.13n | 25.01±1.20a | 66.97±1.27g | ||
WL | 92.36±1.07p | 39.81±1.07d | 40.76±1.07e | 27.24±1.20b | 37.32±1.13c | ||
L2 | BLOCK 2 | FP | 92.06±1.07q | 76.06±1.13g | 82.02±1.07j | 82.67±1.07k | 85.29±1.20l |
AN | 91.29±1.15p | 90.43±1.07mn | 85.03±1.07l | 90.78±1.07no | 79.19±1.20i | ||
AL | 91.08±1.07op | 77.61±1.13h | 46.07±1.13d | 30.72±1.07a | 31.06±1.20a | ||
WL | 90.22±1.22m | 44.49±1.20c | 57.27±1.13f | 46.80±1.07e | 41.78±1.07b | ||
L31 | BLOCK 3 | FP | 90.05±1.07n | 82.63±1.00j | 84.86±1.07k | 79.88±1.07i | 78.51±1.26h |
AN | 85.03±1.07k | 91.46±1.20p | 89.49±1.07m | 90.65±1.07o | 88.20±1.07l | ||
AL | 89.06±1.13m | 91.72±1.20p | 42.81±1.07e | 40.63±1.54d | 38.35±1.13c | ||
WL | 90.91±1.07o | 61.13±1.00f | 62.21±1.07g | 31.92±1.00a | 35.78±1.13b | ||
L33 | BLOCK 4 | FP | 84.81±1.13i | 81.21±1.00g | 85.37±1.07ij | 80.87±1.07g | 76.23±1.20f |
AN | 85.71±1.45j | 91.33±1.07i | 91.25±1.13l | 92.28±1.13m | 89.66±1.07k | ||
AL | 82.20±1.15h | 91.38±1.00l | 63.06±1.00e | 26.00±1.22a | 49.64±1.61d | ||
WL | 82.50±1.13h | 49.29±1.13d | 48.43±1.07c | 39.51±1.00b | 49.03±1.26cd | ||
L52 | BLOCK 5 | FP | 85.29±1.07kl | 76.36±1.20h | 86.87±1.13m | 76.19±1.13h | 84.81±1.13k |
AN | 85.54±1.07l | 78.81±1.07i | 91.21±1.07p | 89.83±1.00o | 91.16±1.30p | ||
AL | 84.86±1.07k | 80.61±1.07j | 41.23±1.07c | 26.64±1.22a | 42.21±1.56d | ||
WL | 87.90±1.00n | 40.50±1.27b | 49.72±1.27g | 48.82±1.15f | 47.96±1.07e | ||
L7 | BLOCK 6 | FP | 93.05±1.13o | 76.53±1.07h | 85.20±1.13j | 82.07±1.07i | 85.84±1.34k |
AN | 93.91±1.07p | 88.12±1.07m | 91.72±1.07n | 87.52±1.13lm | 87.43±1.07l | ||
AL | 86.10±1.00k | 56.11±1.13g | 34.11±1.00a | 40.84±1.07e | 37.15±1.20c | ||
WL | 93.39±1.07op | 37.37±1.07c | 55.38±1.07f | 35.82±1.07b | 38.52±1.75d | ||
Mean±SD(n=3) values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7
Table 12. Percentage variation in DPPH radical scavenging activity during fermentation.
Vegetable | LAB Strains | DPPH variation during fermentation / (%) | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | ||
FP | Un | 0.0 | -23.7 | -14.8 | -14.7 | -7.3 |
L2 | 0.0 | -17.4 | -10.9 | -10.2 | -7.4 | |
L31 | 0.0 | -8.2 | -5.8 | -11.3 | -12.8 | |
L33 | 0.0 | -4.2 | 0.7 | -4.7 | -10.1 | |
L52 | 0.0 | -10.5 | 1.9 | -10.7 | -0.6 | |
L7 | 0.0 | -17.8 | -8.4 | -11.8 | -7.7 | |
AN | Un | 0.0 | -3.2 | -9.3 | -15.8 | -8.5 |
L2 | 0.0 | -0.9 | -6.9 | -0.6 | -13.3 | |
L31 | 0.0 | 7.6 | 5.2 | 6.6 | 3.7 | |
L33 | 0.0 | 6.6 | 6.5 | 7.7 | 4.6 | |
L52 | 0.0 | -7.9 | 6.6 | 5.0 | 6.6 | |
L7 | 0.0 | -6.2 | -2.3 | -6.8 | -6.9 | |
AL | Un | 0.0 | -46.7 | -5.5 | -72.8 | -27.2 |
L2 | 0.0 | -14.8 | -49.4 | -66.3 | -65.9 | |
L31 | 0.0 | 3.0 | -51.9 | -54.4 | -56.9 | |
L33 | 0.0 | 11.2 | -23.3 | -68.4 | -39.6 | |
L52 | 0.0 | -5.0 | -51.4 | -68.6 | -50.3 | |
L7 | 0.0 | -34.8 | -60.4 | -52.6 | -56.9 | |
WL | Un | 0.0 | -56.9 | -55.9 | -70.5 | -59.6 |
L2 | 0.0 | -50.7 | -36.5 | -48.1 | -53.7 | |
L31 | 0.0 | -32.8 | -31.6 | -64.9 | -60.6 | |
L33 | 0.0 | -40.2 | -41.3 | -52.1 | -40.6 | |
L52 | 0.0 | -53.9 | -43.4 | -44.5 | -45.4 | |
L7 | 0.0 | -60.0 | -40.7 | -61.6 | -58.8 | |
Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strain L52=L. plantarum L52, Strain L7=L. pentosusL7 | ||||||
3.8. Effect of lactic acid bacteria strains on the ferric reducing antioxidant power activity
Fermentation significantly(p≤0.05) influenced the FRAP activity of all the AILVs fermented with the different LAB strains under study (Table 13). There was a general increase in FRAP activity in AN, FP, AL and WL fermented with all LAB strains from day 0 to day 3. However, AN leaves fermented with L. mesenteroidesL33 and L. plantarumL31 showed the highest FRAP activity (5433.34 ± 0.00 mg/100g and 5100.00 ± 0.00 mg/100g) respectively, at 3 days fermentation. The frequency at which the FRAP activity of the AILVs changed over the 4 days fermentation period is shown in Table 14. The study revealed that FRAP activity in AN, WL and FP fermented with L. mesenteroidesL33 rises by 61.1%,15.2% and 106.9% at 4 to 2 days of fermentation, respectively. Hence L. mesenteroidesL33 showed the best impact in enhancing the FRAP activity in the vegetables as compared to strains L. plantarumL52, L. plantarumL31, L. mesenteroidesL2 and L. pentosusL7. The increase in antioxidant activity can be attributed to the release of phenolic compounds during fermentation. A similar trend was observed by [9] who found that fermentation for 3 days by the LAB strains L. plantarum (75) and L. plantarum(17a) increased the FRAP antioxidant activity of African nightshade leaves by 11.9% and 7.1%, respectively, when compared to the unfermented leaves. In addition, [32, 38-39] recorded significant increases in FRAP activity of LAB strains fermented wolfberry juice, tempe (fermented rice) and fermented cereals (buckwheat, wheat germ, barley and rye) respectively when compared to the unfermented controls. However, Kaprasob et al., [34] showed a decrease in antioxidant activity in fermented cashew apple juice and explained that this could be due to oxidation of phenolic compounds. Numerous studies have underlined a beneficial health effect for antioxidant-rich foods, such as reducing the risk of non-communicable diseases and premature ageing [40].
Table 13. Influence of fermentation by lactic bacteria strain on ferric reducing antioxidant power activity of the vegetables.
LAB strains | Blocks | Sample | Fermentation time/Days | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | |||
Un | BLOCK 1 | FP | 1534.67±1.15i | 1492.22±1.92h | 1673.11±0.77l | 1725.56±1.93m | 1946.67±0.00p |
AN | 3132.22±1.92r | 1372.22±1.92g | 3444.44±1.92s | 2620.00±0.00q | 1907.78±1.92o | ||
AL | 1577.78±1.92k | 1130.44±0.77a | 1554.89±0.77j | 1731.56±1.02n | 1294.44±1.92f | ||
WL | 1371.56±1.54g | 1167.56±0.38c | 1146.22±0.38b | 1271.33±0.67e | 1232.22±1.92d | ||
L2 | BLOCK 2 | FP | 1492.22±0.38i | 1230.67±0.00d | 2044.44±1.92m | 1806.67±0.00l | 2112.22±1.92 |
AN | 3107.78±1.92p | 2111.11±1.92n | 3406.67±0.00r | 3200.00±0.00q | 2471.11±3.85o | ||
AL | 1545.56±1.92j | 1233.33±0.00d | 1052.22±0.77b | 1431.78±0.39h | 1338.89±1.92e | ||
WL | 1680.22±0.77k | 1039.33±0.67a | 1356.00±0.00f | 1415.78±1.02g | 1143.33±0.00c | ||
L31 | BLOCK 3 | FP | 1454.44±1.92h | 1108.89±1.92b | 2282.22±1.92m | 1940.00±37.56l | 2327.78±1.92n |
AN | 3037.78±1.93q | 2660.00±0.00o | 4814.44±13.47r | 5100.00±0.00s | 2895.56±1.92p | ||
AL | 1444.45±9.62g | 1741.11±1.92j | 1810.22±0.38k | 1062.22±0.77a | 1115.33±0.00c | ||
WL | 1232.89±1.02e | 1671.11±1.92i | 1284.09±3.62f | 1182.67±0.67d | 1435.11±1.02g | ||
L33 | BLOCK 4 | FP | 1230.00±0.00c | 1211.11±1.92b | 2544.44±1.92o | 2295.56±1.93n | 1914.44±1.92l |
AN | 3371.67±1.67p | 2166.67±0.00m | 4818.89±1.92r | 5433.34±0.00s | 4014.44±3.85q | ||
AL | 1765.56±1.92j | 1826.67±0.00k | 1517.33±0.00h | 1620.67±0.67i | 1327.78±1.54f | ||
WL | 1206.67±1.15b | 1013.33±0.67a | 1288.00±0.67d | 1308.89±0.39e | 1389.56±0.38g | ||
L52 | BLOCK 5 | FP | 1644.00±0.00i | 1324.44±1.19g | 2203.33±0.00n | 2683.33±3.33p | 2172.22±1.92m |
AN | 3701.11±6.94q | 1926.67±3.33l | 3764.77±4.13r | 4231.11±1.92s | 2501.11±1.92o | ||
AL | 1700.00±0.00k | 1050.67±0.67b | 1002.22±0.38a | 1087.11±0.77c | 1327.78±1.92g | ||
WL | 1668.89±1.92j | 1358.22±0.38h | 1237.11±0.38d | 1303.33±0.67f | 1294.44±1.92e | ||
L7 | BLOCK 6 | FP | 1412.22±0.77f | 1211.11±1.92b | 2090.00±0.00i | 1938.89±1.92h | 2202.22±1.92j |
AN | 3086.67±3.33m | 2218.89±1.93j | 3581.11±1.02n | 2300.00±0.00k | 2651.11±1.92l | ||
AL | 1695.56±1.92g | 1064.00±23.69a | 1252.00±1.67cd | 1027.78±0.38a | 1346.67±0.00e | ||
WL | 1668.891.92g | 1261.33±0.00d | 1216.67±0.00bc | 1240.44±1.02bcd | 1223.33±0.00bc | ||
Mean±SD values within the same BLOCK followed by the same superscripts are not significantly different at p≤0.05. Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strainL52=L. plantarum L52, Strain L7=L. pentosusL7 | |||||||
Table 14. percentage variation in ferric reducing antioxidant power activity during fermentation.
Vegetable | LAB Strains | FRAP variation during fermentation / (%) | ||||
Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | ||
FP | Un | 0.0 | -2.8 | 9.0 | 12.4 | 26.8 |
L2 | 0.0 | -17.5 | 37.0 | 21.1 | 41.5 | |
L31 | 0.0 | -23.8 | 56.9 | 33.4 | 60.0 | |
L33 | 0.0 | -1.5 | 106.9 | 86.6 | 55.6 | |
L52 | 0.0 | -19.4 | 34.0 | 63.2 | 32.1 | |
L7 | 0.0 | -14.2 | 48.0 | 37.3 | 55.9 | |
AN | Un | 0.0 | -56.2 | 10.0 | -16.4 | -39.1 |
L2 | 0.0 | -32.1 | 9.6 | 3.0 | -20.5 | |
L31 | 0.0 | -12.4 | 58.5 | 67.9 | -4.7 | |
L33 | 0.0 | -35.7 | 42.9 | 61.1 | 19.1 | |
L52 | 0.0 | -47.9 | 1.7 | 14.3 | -32.4 | |
L7 | 0.0 | -28.1 | 16.0 | -25.5 | -14.1 | |
AL | Un | 0.0 | -28.4 | -1.5 | 9.7 | -18.0 |
L2 | 0.0 | -20.2 | -31.9 | -7.4 | -13.4 | |
L31 | 0.0 | 20.5 | 25.3 | -26.5 | -22.8 | |
L33 | 0.0 | 3.5 | -14.1 | -8.2 | -24.8 | |
L52 | 0.0 | -38.2 | -41.0 | -36.1 | -21.9 | |
L7 | 0.0 | -37.2 | -26.2 | -39.4 | -20.6 | |
WL | Un | 0.0 | -14.9 | -16.4 | -7.3 | -10.2 |
L2 | 0.0 | -38.1 | -19.3 | -15.7 | -32.0 | |
L31 | 0.0 | 35.5 | 17.6 | -4.1 | 16.4 | |
L33 | 0.0 | -16.0 | 6.7 | 8.5 | 15.2 | |
L52 | 0.0 | -18.6 | -25.9 | -21.9 | -22.4 | |
L7 | 0.0 | -24.4 | -27.1 | -25.7 | -26.7 | |
Un-control strain L2= L. mesenteroides, strain L3=L. plantarumL31, strain L33=L. mesenteroides, strain L52=L. plantarum L52, Strain L7=L. pentosusL | ||||||
4. Conclusions
This work provides a study of four fermented AILVs (AN, FP, AL and WL) by lactic acid bacteria isolated from fresh water leaf (L. pentosusL7), fluted pumpkin leaf (L. mesenteroidesL31), African nightshade (L. plantarumL31 and L. plantarumL52) and Amaranthus leaf (L. mesenteroidesL2). Vegetables inoculated with L. plantarumL52 demonstrated the most significant reduction in pH from 6.39 to 3.82. Starter culture-inoculated L. plantarumL31 recorded the highest effect on total phenolic content and TAC while L. mesenteroidesL33 inoculated vegetables recorded the highest DPPH radical scavenging and FRAP activities. Based on the present study, fermenting AILVs for 3 days yielded the most significant improvements in phenolic content and antioxidant properties. Thus, the results of this study could contribute to lactic acid bacteria fermentation from consumed fresh African indigenous leafy vegetables and could help to improve their phytochemical content and antioxidant capacity. For more detailed information, more studies are needed to be carried out with other assays and other studied conditions (at different temperatures: 30℃, 35℃, 40℃, 45℃ and at different starter concentrations).
Authors’ contributions
Conceptualization, data curation, funding acquisition, investigation, methodology, writing original draft, N.C.E.; conceptualization, project administration, resources, supervision, review and editing, F.D.R.; formal analysis, methodology, software, validation, visualization; A.D.T.K.; data curation, methodology, resources, review and editing, O.E.R.B.; formal analysis. Investigation, methodology, review and editing, M.K.C.H; formal analysis, methodology, review and editing, N.M.B.; conceptualization, project administration, resources, supervision, validation, visualization, review and editing, E.R.A.
Acknowledgements
The authors would like to thank the laboratory of Food Science and Metabolism (LabSam), Department of Biochemistry, University of Yaounde 1; the Microbiology laboratory (LABO180), Department of Microbiology, University of Yaounde 1 and Inqaba Biotec Central Africa, Yaounde, Africa’s Genomics Centre, for all the laboratory assistance that let to realization of this study.
Funding
The work was carried out with the authors’ own funding. This study did not receive any external funding.
Availability of data and materials
All data will be made available on request according to the Journal policy.
Conflicts of interest
The authors declare no conflicts of interest.
References
1. | Abukutsa-Onyango, M. Strategic repositioning of African indigenous vegetables in the horticulture sector. In Proceedings of the Second RUFORUM Biennial Meeting, Entebbe, Uganda. 2014, 1413–1419. https://doi.org/10.1002/9781118460566.ch25 |
2. | Grubben, G.J.H. Communication in the Department of Agricultural Research, No. 67. Amsterdam, The Netherlands: Royal Tropical Institute, 1976. |
3. | Ihekoronye, A.I.; Ngoddy, P.O. Integrated food science and technology. New York: Macmilian Publishers, 1985. https://doi.org/10.1016/b978-0-12-054805-7.50001-4 |
4. | Oyenuga, V.A. ; Fetuga, B.L. The apparent digestibility of nutrients and energy values of some oilseed meals and three commonly used cereal grains fed to pigs. East Afr. Agri. Forestry J. 1975, 9(1), 63–110. https://doi.org/10.1080/00128325.1975.11662759 |
5. | Yang, R.Y.; Keding, G.B. Nutritional contributions of important African indigenous vegetables. African indigenous vegetables in urban agriculture. In C. M. Shackleton, M. W. Pasquini & A. W. Dresche (Eds.), African indigenous vegetables in urban agriculture. 105–143, 2010. https://doi.org/10.1002/ldr.967 |
6. | Traoré, K.; Parkouda, C.; Savadogo, A.; Ba/Hama, F.; Kamga, R.; Traoré, Y. Effect of processing methods on the nutritional content of three traditional vegetables leaves: Amaranth, black nightshade and jute mallow. Food Sci. Nutr. 2017, 5, 1139–1144. https://doi.org/10.1002/fsn3.504 |
7. | Bayi, J.; Ngah, E.; Megueni, E. Inventory of the biodiversity of traditional vegetables consumed by the people of the Nyong and Kelle Division: Cameroon. GSC Bio. Pharm. Sci. 2020, 10(3), 53-68. https://doi.org/10.30574/gscbps.2020.10.3.0052 |
8. | Owade, J.O.; Abong’, G.O.; Okoth, M.W.; Mwang’ombe, A.W.; Jobor, J.O. Comparative profiling of lactic acid bacteria isolates in optimized and spontaneous fermentation of cowpea leaves. Food Sci. Nutr. 2021, 9, 1651–1664. https://doi.org/10.1002/fsn3.2140 |
9. | Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effects of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African Nightshade. Microorganisms. 2020, 8, 1324. https://doi.org/10.3390/microorganisms8091324 |
10. | Irakoze, M.L.; Wafula, E.N.; Owaga, E.E. Effect of lactic acid fermentation on phytochemical content, antioxidant capacity, sensory acceptability and microbial safety of African black nightshade and African spider plant vegetables. Bacteria. 2023, 2, 48–59. https:// doi.org/10.3390/bacteria2010004 |
11. | Odongo, G.A.; Schlotz, N.; Baldermann, S.; Neugart, S.; Huyskens-Keil, S.; Ngwene, B.; Trierweiler, B.; Schreiner, M.; Lamy, E. African nightshade (Solanum scabrum Mill.): Impact of cultivation and plant processing on its health promoting potential as determined in a human liver cell model. Nutrients. 2018, 10, 1532. https://doi.org/10.3390/nu10101532 |
12. | Wu, Y.; Ye, Z.; Feng, P.; Li, R.; Chen, X.; Tian, X.; et al. Limosilactobacillus fermentum JL-3 isolated from“Jiangshui” ameliorates hyperuricemia by degrading uric acid. Gut Microbes. 2021, 13, 1–18. https://doi.org/10.1080/19490976.2021.1897211 |
13. | Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutri. 2014, 54(9), 1241-1251. https://doi.org/10.1080/10408398.2011.621169 |
14. | Ruiz Rodríguez L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in northern Argentina. Front. Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.01091 |
15. | Ibinabo, T.I.; Wafula, E.N.; Josiah, K.; Julius, M.M. Phenotypic and genotypic characterization of lactic acid bacteria isolated from spontaneously fermented vegetable amaranth. Afr. J. Food Sci. 2021, 15, 254–261. https://doi.org/10.5897/ajfs2021.2107 |
16. | Wafula, E.N.; Kuja, J.O.; Wekesa, T.B.; Wanjala, P.M. Isolation and identification of autochthonous lactic acid bacteria from commonly consumed African indigenous leafy vegetables in Kenya. Bacteria. 2023, 2, 1–20. https://doi.org/10.3390/ bacteria2010001 |
17. | Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019 https://doi.org/10.1155/2019/7179514 |
18. | Ilesami, T.M.; Eniola, K.I.T. Isolation and antimicrobial activity of lactic acid bacteria isolated from pineapple and watermelon. GSC Bio. Pharm. Sci. 2023, 23(2), 148-154. http://doi.org/10.30574/gscbps.2023.23.2.0173 |
19. | Lane, D.J. 16S/23S rRNA sequencing. Nucleic acids techniques in bacteria systematics. E. Stackebrandt and M. Goodfellow, eds. New York, NY John Wiley and sons. 1991, 115-175. https://doi.org/10.1002/jobm.3620310616 |
20. | Woods, D.C.; Lewis, S.M. Design of Experiments for Screening. Southampton Statistical Sciences Research Institute. 2015, 1-32. https://doi.org/10.1007/978-3-319-11259-6_33-1 |
21. | Wafula, E. Effects of postharvest-processing technologies on the safety and quality of African indigenous leafy vegetables. PhD Thesis. 2017, 37-56. |
22. | Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010, 15, 7313-7352. https://doi.org/10.3390/molecules15107313 |
23. | Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008. 56, 9732–9739. https://doi.org/10.1021/jf801069s |
24. | Vinson, J.A.; Yong, H.; Xuehui, S.; Ligia, Z. Phenol antioxidant quantity and quality in foods : vegetables. J. Agric. Food Chem. 1998, 46, 3630-3634. https://doi.org/10.1021/jf980295o |
25. | Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochem. 2008, 69(8), 1732-1738. https://doi.org/10.1016/j.phytochem.2008.02.014 |
26. | Benzie, F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of « Antioxidant Power ». The FRAP assay. Analyt. Biochem. 1996, 239, 70-76. https://doi.org/10.1006/abio.1996.0292 |
27. | Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. J. Analyt. Biochem. 1999, 269, 337–341. https://doi.org/10.1006/abio.1999.4019 |
28. | Njong,C.E.; Manga, G.G.; Sonchieu, J.; Ejoh, R.A. Impact of different household cooking methods on the nutritional composition, total phenolic content and invitro antioxidant activities of African nightshade (Solanum scabrum) and vegetable amaranth (Amaranthus hybridis). Int. J. Food Sci. 2025, 7, 1 – 22. https://doi.org/10.47941/jfs.2459 |
29. | Adebo, O.A.; Medina-Meza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules. 2020, 25, 927. https://doi.org/10.3390/molecules25040927 |
30. | Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G.; et al. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. https://doi.org/10.1016/j.envres.2021.111531 |
31. | Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.H.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. https://doi.org/10.1002/jsfa.10272 |
32. | Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants. 2021, 10, 2004. https://doi.org/10.3390/antiox10122004 |
33. | Abduxukur, D.; Tursuntay, A.; Zhu, X.; Wang, X.; Rahman, N. Antioxidant capacity of lactic acid bacteria and yeasts from Xinjiang traditional fermented dairy products. Fermentation. 2023, 9, 639. https://doi.org/10.3390/ fermentation9070639 |
34. | Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. https://doi.org/10.1016/j.procbio.2017.05.019 |
35. | Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; Pablo López-Gómez, J. Multi-product lactic acid bacteria fermentations: A Review. Fermentation. 2020, 6, 23. https://doi.org/10.3390/fermentation6010023 |
36. | Gomez-Zavaglia, A.; De Los Angeles Serradell, M.; Ozogul, F.; Tamang, J.P.; Shin, D.-H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. https://doi.org/10.3389/fmicb.2016.00578 |
37. | Wijayanti, E.D.; Setiawan, N.C.E.; Christi, J.P. Effect of lactic acid fermentation on total phenolic content and antioxidant activity of fig fruit juice (Ficus carica). Atlantis Press. 2017, 2, 282–289. https://doi.org/10.2991/hsic-17.2017.44 |
38. | Dey, T.B.; Kuhad, R.C. Upgrading the antioxidant potential of cereals by their fungal fermentation under solid-state cultivation conditions. Lett. Appl. Microbiol. 2014, 59(5). https://doi.org/10.1111/lam.12300 |
39. | Ðordevic, T.M.; Šiler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. https://doi.org/10.1016/j.foodchem.2009.07.049 |
40. | Grosso, G. Dietary antioxidants and prevention of non-communicable diseases. Antioxidants. 2018, 7, 94 https://doi.org/10.3390/antiox7070094 |
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
This study aimed to identify lactic acid bacteria (LAB) from fresh African indigenous leafy vegetables (AILVs) consumed in Cameroon and screen them for total phenolic content and antioxidant capacity upon fermentation. The LAB were isolated from four AILVs; Solanum scabrum (African nightshade, AN), Telfeiria occidentalis (fluted pumpkin, FP), Amaranthus hybridus (Amaranthus vegetable, AL) and Talinum triangulaire (Waterleaf, WL). The LAB isolates were identified biochemically and molecularly. The LAB strains were then preserved in 20% glycerol at -20°C and later reactivated to carry out fermentation of the AILVs. Fermentation trials using a systematic replicate screening design, were conducted at 27°C with each LAB strain over a period of 0-4 days. The pH, total phenolics (FrP and BF), and antioxidant activities; ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl(DPPH) and total antioxidant capacity (TAC) were assessed using standard methods. The LAB isolates were identified by 16S rRNA gene sequencing as Lactiplantibacillus plantarumL52, Lactiplantibacillus plantarumL31, Leuconostoc mesenteroidesL33, Leuconostoc mesenteroidesL2 and Lactiplantibacillus pentosusL7. Fermentation with L. plantarumL52 strain significantly (p ≤ 0.05) decreased the pH from 6.39±0.006 to 3.82±0.006 in the AILVs. L. plantarumL31 fermented AILVs significantly increased (by 56.6% and 140.2%) FrP content and TAC activity respectively while L. mesenteroidesL33 significantly recorded the highest percentage DPPH (7.7%) and FRAP (106.9%) activities after 4 days. The most improved active metabolite trend was observed at 3 days of fermentation after screening. Overall, L. plantarumL31 and L. mesenteroidesL33 were the most functionally effective strains, and AN emerged as the most potent substrate among the vegetables. examined.
Abstract Keywords
Lactic acid bacteria, African indigenous leafy vegetables, fermentation, antioxidant activity, phenolic content.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


